Abstract
Background
Von Willebrand disease (VWD) is the most common inherited bleeding disorder. However, studies of hospitalisation patterns with replacement treatment are scarce.
Objectives
The aim of this study was to investigate the current therapeutic management of VWD and determine the key drivers of coagulation factor uses in patients during hospitalisation.
Methods
Hopscotch-WILL was a multi-centric retrospective study conducted over a 48-month period in any patients with VWD. The data were collected from the BERHLINGO Research Database and the French Hospital database.
Results
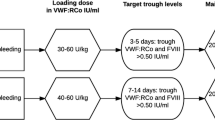
A total of 988 patients were included; 153 patients (15%) were hospitalised during 293 stays requiring treatment with von Willebrand factor (VWF) concentrates—pure or in association with Factor VIII (FVIII). Their median basal concentrations of VWF and FVIII were significantly lower than in untreated patients: VWF antigen < 30 IU/dL, VWF activity < 20 IU/dL and FVIII:C < 40 IU/dL. The median (interquartile range) concentrate consumption was similar between highly purified VWF or VWF combined with FVIII (72 [110] vs 57 [89] IU/kg/stay, p = 0.154). The use of VWF was highly heterogeneous by VWD type; type 3 had a particularly high impact on VWF consumption in non-surgical situations. The main admissions were for ear/nose/throat, hepato-gastroenterology, and trauma/orthopaedic conditions, besides gynaecological-obstetric causes in women.
Conclusions
The use of VWF concentrates is mostly influenced by low basal levels of VWF and FVIII, but also by VWD type or the cause for hospitalisation. These results could inform future studies of newly released recombinant VWF.



Similar content being viewed by others
References
Sharma R, Flood VH. Advances in the diagnosis and treatment of Von Willebrand disease. Blood. 2017;130:2386–91. https://doi.org/10.1182/blood-2017-05-782029.
Fressinaud É. Clinical, biological and molecular diagnosis. Hématologie. 2014;30–49. DOI: https://doi.org/10.1684/hma.2014.0885
Goodeve A, James P. von Willebrand disease. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2018 May 17]. http://www.ncbi.nlm.nih.gov/books/NBK7014/. Bookshelf ID: NBK7014.
Swami A, Kaur V. von Willebrand disease: a concise review and update for the practicing physician. Clin Appl Thromb Hemost. 2017;23:900–10. https://doi.org/10.1177/1076029616675969.
Boisseau P. Molecular genetic techniques. Hématologie. 2014. https://doi.org/10.1684/hma.2014.0884.
Sadler JE, Mannucci PM, Berntorp E, Bochkov N, Boulyjenkov V, Ginsburg D, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000;84:160–74. https://doi.org/10.1055/s-0037-1613992.
Holm E, Carlsson KS, Lövdahl S, Lail AE, Abshire TC, Berntorp E. Bleeding-related hospitalization in patients with von Willebrand disease and the impact of prophylaxis: Results from national registers in Sweden compared with normal controls and participants in the von Willebrand Disease Prophylaxis Network. Haemophilia Internet. 2018. https://doi.org/10.1111/hae.13473.
Heijdra JM, Cnossen MH, Leebeek FWG. Current and Emerging Options for the Management of Inherited von Willebrand Disease. Drugs. 2017;77:1531–47. https://doi.org/10.1007/s40265-017-0793-2.
CRMW. Protocole National de Diagnostic et de Soins (PNDS) : Maladie de Willebrand type 3 [Internet]. HAS; 2021 Nov. https://www.has-sante.fr/upload/docs/application/pdf/2022-01/pnds_type_3_3_11_2021.pdf.
Lethagen S, Kyrle PA, Castaman G, Haertel S, Mannucci PM, FOR THE HAEMATE P® SURGICAL STUDY GROUP. von Willebrand factor/factor VIII concentrate (Haemate®-P) dosing based on pharmacokinetics: a prospective multicenter trial in elective surgery. J Thromb Haemost. 2007;5:1420–30. https://doi.org/10.1111/j.1538-7836.2007.02588.x.
Gill JC, Shapiro A, Valentino LA, Bernstein J, Friedman C, Nichols WL, et al. von Willebrand factor/factor VIII concentrate (Humate®-P) for management of elective surgery in adults and children with von Willebrand disease: VWF/FVIII concentrate in elective surgery. Haemophilia. 2011;17:895–905. https://doi.org/10.1111/j.1365-2516.2011.02534.x.
Hazendonk HCAM, Heijdra JM, de Jager NCB, Veerman HC, Boender J, van Moort I, et al. Analysis of current perioperative management with Haemate®-P/Humate®-P in von Willebrand disease: Identifying the need for personalized treatment. Haemophilia [Internet]. 2018. https://doi.org/10.1111/hae.13451.
Miesbach W, Krekeler S, Wolf Z, Seifried E. Clinical use of Haemate®-P in von Willebrand disease: a 25-year retrospective observational study. Thromb Res. 2015;135:479–84. https://doi.org/10.1016/j.thromres.2014.12.017.
Windyga J, von Depka-Prondzinski M, the European Wilate® Study Group. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (WILATE®) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost. 2011;105:1072–9. https://doi.org/10.1160/TH10-10-0631.
Borel-Derlon A, Federici AB, Roussel-Robert V, Goudemand J, Lee CA, Scharrer I, et al. Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin®): a prospective study of 50 patients. J Thromb Haemost. 2007;5:1115–24. https://doi.org/10.1111/j.1538-7836.2007.02562.
Srivastava A, Serban M, Werner S, Schwartz BA, Kessler CM, the Wonders Study Investigators. Efficacy and safety of a VWF/FVIII concentrate (WILATE®) in inherited von Willebrand disease patients undergoing surgical procedures. Haemophilia. 2017;23:264–72. https://doi.org/10.1111/hae.13106.
Kulikov AU, Babiy VV. Pharmacoeconomic analysis of von Willebrand factor/blood clotting factor VIII Concentrate (WILATE®) in the management of patients with Von Willebrand disease. Pharmacoecon Theory Pract. 2015;3:74–81. https://doi.org/10.30809/phe.2.2015.4.
Cipolla A, Cultrera D, Teruzzi C, Mantuano M. Cost-consequences analysis of the long-term prophylaxis in a type 1 Von Willebrand disease patient with recurrent bleedings in Italy. Value Health. 2014;17:A530. https://doi.org/10.1016/j.jval.2014.08.1681.
Teruzzi C, Schinco P, Cultrera D, Valeri F, Borchiellini A, Mantuano M, et al. Cost-consequence analysis of long-term prophylaxis in the treatment of von Willebrand disease in the Italian context. Clinicoecon Outcomes Res. 2014. https://doi.org/10.2147/CEOR.S71892.
Schinco P, Castaman G, Coppola A, Cultrera D, Ettorre C, Giuffrida AC, et al. Current challenges in the diagnosis and management of patients with inherited von Willebrand’s disease in Italy: an Expert Meeting Report on the diagnosis and surgical and secondary long-term prophylaxis. Blood Transfus [Internet]. 2017 [cited 2018 May 16]; http://www.bloodtransfusion.it/articolosing.aspx?id=000935. DOI: https://doi.org/10.2450/2017.0354-16.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X.
INSEE R. INSEE - Évolution générale de la situation démographique par département et région - Séries depuis 1990 (Bretagne & Pays-de-Loire) [Internet]. 2020 [cited 2021 Dec 16]. https://www.insee.fr/fr/statistiques/fichier/4503164/p1d.xlsx.
Dolan G, Makris M, Bolton-Maggs PHB, Rowell JA. Enhancing haemophilia care through registries. Haemophilia. 2014;20:121–9. https://doi.org/10.1111/hae.12406.
Boulanger V, Schlemmer M, Rossov S, Seebald A, Gavin P. Establishing patient registries for rare diseases: rationale and challenges. Pharm Med. 2020;34:185–90. https://doi.org/10.1007/s40290-020-00332-1.
Jonker CJ, Bakker E, Kurz X, Plueschke K. Contribution of patient registries to regulatory decision making on rare diseases medicinal products in Europe. Front Pharmacol. 2022;13: 924648. https://doi.org/10.3389/fphar.2022.924648.
Veyradier A, Boisseau P, Fressinaud E, Caron C, Ternisien C, Giraud M, et al. A laboratory phenotype/genotype correlation of 1167 French patients from 670 families with von Willebrand Disease: a new epidemiologic picture. Medicine (Baltimore). 2016;95: e3038. https://doi.org/10.1097/MD.0000000000003038.
Federici A, Bucciarelli P, Castaman G, Baronciani L, Canciani M, Mazzucconi M, et al. Management of inherited von Willebrand disease in italy: results from the retrospective study on 1234 patients. Semin Thromb Hemost. 2011;37:511–21. https://doi.org/10.1055/s-0031-1281037.
Batlle J, Pérez-Rodríguez A, Corrales I, López-Fernández MF, Rodríguez-Trillo Á, Lourés E, et al. Molecular and clinical profile of von Willebrand disease in Spain (PCM–EVW–ES): proposal for a new diagnostic paradigm. Thromb Haemost. 2016;115:40–50. https://doi.org/10.1160/TH15-04-0282.
Goudemand J, Bridey F, Claeyssens S, Itzhar-Baïkian N, Harroche A, Desprez D, et al. Management of von Willebrand disease with a factor VIII-poor von Willebrand factor concentrate: Results from a prospective observational post-marketing study. J Thromb Haemost. 2020;18:1922–33. https://doi.org/10.1111/jth.14928.
Goudemand N, Ounnoughene S. Clinical management of patients with von Willebrand’s disease with a VHP vWF concentrate: the French experience: treatment of von Willebrand’s disease. Haemophilia. 1998;4:48–52. https://doi.org/10.1046/j.1365-2516.1998.0040s3048.x.
Rugeri L, d’Oiron R, Harroche A, Proulle V, Mourey G, De Raucourt E, Desprez D, Baikian NI, Petesch BP, Borel-Derlon A, Combe S, Frotscher B, Hassoun A, Catovic H, Bracquart D, Trossaërt M. Effectiveness and safety of hFVIII/VWF concentrate (Voncento®) in patients with inherited von Willebrand disease requiring surgical procedures: the OPALE multicentre observational study. Blood Transfus. 2021;19(2):152–7. https://doi.org/10.2450/2020.0246-20. (Epub 2020 Nov 27. PMID: 33263522; PMCID: PMC7925218).
Federici AB, Barillari G, Zanon E, Mazzucconi MG, Musso R, Targhetta R, et al. Efficacy and safety of highly purified, doubly virus-inactivated VWF/FVIII concentrates in inherited von Willebrand’s disease: results of an Italian cohort study on 120 patients characterized by bleeding severity score. Haemophilia. 2010;16:101–10. https://doi.org/10.1111/j.1365-2516.2009.02088.x.
Swallow E, Marden JR, Billmyer E, Yim E, Sun SX. Burden of illness and treatment patterns among patients with von willebrand disease in US Clinical Practice. Clin Appl Thromb Hemost. 2023;29:107602962311770.
ATIH. ENC MCO. Analyse de l’ensemble des séjours : coût moyen d’un séjour en 2018 [Internet]. ATIH; 2018. https://www.scansante.fr/applications/enc-mco/submit?snatnav=&annee=2018§eur=dgf&type_activite=ghs&cmd=&souscmd=&racine=&ghm=TOTAL&mbout=dummy&num_selection=TOTAL&type_selection=ghm&zip=non.
Jacobs K, Roman E, Lambert J, Moke L, Scheys L, Kesteloot K, et al. Variability drivers of treatment costs in hospitals: A systematic review. Health Policy. 2022;126:75–86. https://doi.org/10.1016/j.healthpol.2021.12.004.
Duburcq A, Courouve L, Mercier C, Scherman D, Lévy P, Bahi-Buisson N, et al. Coût des maladies rares en France en 2017: une analyse des données du Système national des données de santé sur cinq pathologies. Rev Epidemiol Sante Publique. 2020;68:S76–7. https://doi.org/10.1016/j.respe.2020.04.028.
Colin C, Couray-Targe S, Loïc G. PMSI : Quelles comparaisons possibles et impossibles ? ADSP. 2000; Organisation, décision et financement du système de soins:53–5. ISSN: 1243-275X
Acknowledgements
The authors thank the patients and their families for participating in this study. This study was conducted within the French network of University Hospitals HUGO (Hôpitaux Universitaires du Grand Ouest). The authors would like to thank Ms Jill Rupnow (Kokopelli SARL, Paris, France) for providing medical writing services; these services were funded through the study budget. Lastly, the authors thank the members of the BERHLINGO consortium for their daily involvement in the collection of high-quality data and for the attention they devoted to this research project. The BERHLINGO consortium: Sabrina Cochennec, Magalie Cornec, Guillaume Drugmanne, Hubert Galinat, Isabelle Gouin, Estelle Leroy, Fabienne Nedelec-Gac.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was funded by Baxalta GmbH, a wholly subsidiary of Takeda Pharmaceutical Company Limited, as part of an investigator-initiated research project (IIR-FXX-002472). Baxalta GmbH played no role in the design and conduct of the study/collection, management, analysis, or interpretation of the data/preparation, writing, review, or approval of the manuscript.
Conflicts of Interest
Valérie Horvais, Philippe Beurrier, Vincent Cussac, Brigitte Pan-Petesch, Solène Schirr-Bonnans, Johann Rose, Sophie Bayart, Catherine Ternisien, Marc Fouassier, Marianne Sigaud, Antoine Babuty and Nicolas Drillaud declare that they have no conflict of interest. Benoît Guillet received research grants from CSL Behring and consulting fees from CSL Behring/LFB/Takeda. Marc Trossaërt received funding from Baxalta GmbH for the Hopscotch Will I study.
Data Availability
The data underlying the findings of this study are not openly available because they are sensitive. They can be obtained from the corresponding author upon reasonable request. The data are stored in a controlled access facility at Nantes University Hospital.
Code Availability
Not applicable.
Ethics Statement
The Institutional Ethics Committee approved the study protocol on 3 April 2019 (Groupe Nantais d'Ethique dans le Domaine de la Santé, GNEDS).
Consent to Participate
The patient's non-objection was required for inclusion in the study. No written consent was required.
Consent for Publication
Not applicable.
Author Contributions
Valérie Horvais, Solène Schirr-Bonnans and Marc Trossaërt contributed to the conception and design of the study. All of the authors contributed to the preparation of the materials and collection of the data. The analyses were performed by Valérie Horvais and Solène Schirr-Bonnans. The first draft of the manuscript was written by Valérie Horvais and all authors commented on all intermediary versions of the manuscript. All authors read and approved the final manuscript.
Additional information
The members of the BERHLINGO consortium involved in the study are listed in the “Acknowledgements" section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horvais, V., Beurrier, P., Cussac, V. et al. Key Drivers of Coagulation Factor Use in Von Willebrand Disease During Hospitalization: An Overview of the French BERHLINGO Cohort. Clin Drug Investig 44, 35–49 (2024). https://doi.org/10.1007/s40261-023-01323-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01323-1