Abstract
Background
Previous studies have shown conflicting observations regarding the correlation between sodium-glucose-cotransporter-2 inhibitors (SGLT2i) and acute renal failure. Although wide use has contributed to the accumulation of safety information on SGLT2i, the examination of the countries reporting cases of SGLT2i use and influence of concomitant drugs has been insufficient in studies using spontaneous adverse event reporting databases.
Objective
We aimed to re-examine the correlation between SGLT2i and acute renal failure using the latest United States Food and Drug Administration’s Adverse Event Reporting System (FAERS) records and to conduct a stratified analysis for the reporting countries (Japan or other countries), as well as the concomitant use of drugs such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) with SGLT2i.
Patients and Methods
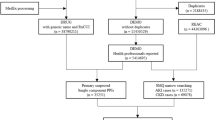
The reporting odds ratio (ROR) and 95% confidence interval (CI) for cases recorded on FAERS from January 2013 to March 2020 were calculated. We then limited the cases to patients using SGLT2i and receiving treatment for diabetes mellitus and then calculated the ROR. A stratified analysis was performed for reporting countries (Japan or other countries), and the presence or absence of concomitant use of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) to examine their influence on the correlation between SGLT2i and acute renal failure.
Results
Of the 5,337,069 cases of adverse events recorded on FAERS, 410,569 were cases in which patients had received treatment for diabetes. The ROR for SGLT2i calculated from the total analysis subjects was 4.16 (95% CI 4.01–4.31), suggesting its correlation with acute renal failure. Similar results were obtained for the cases in which patients had received treatment for diabetes. However, the stratified analysis of these diabetes-treatment cases for reporting countries showed no correlation between SGLT2i and acute renal failure in cases reported in Japan with ROR 0.58 (95% CI 0.49–0.69). In contrast, a correlation was suggested in cases reported in countries other than Japan with ROR 1.91 (95% CI 1.83–1.98). Moreover, the stratified analysis for the concomitant use of an ACEi or ARB showed that the ROR tended to be low in the cases with one of these drugs.
Conclusion
Examination with the signal detection method using FAERS suggested the correlation between SGLT2i and the onset of acute renal failure. However, when focusing on the cases reported in Japan, such a correlation was not suggested. In addition, this study indicated that the signal of acute renal failure tends to be reduced in cases with the concomitant use of either an ACEi or ARB. Through this study we suggest that patients should be closely monitored when they take SGLT2i without an ACEi or ARB.

Similar content being viewed by others
References
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). 14 Jun 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed 9 July 2020.
The Committee on the Proper Use of SI. Recommendations on the proper use of SGLT2 inhibitors. Diabetology International. 2019 2019/11/15.
PMDA. PMDA revises a label tofogliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/03.pdf. Accessed 9 July 2020.
PMDA. PMDA revises labels ipragliflozin, dapagliflozin, luseogliflozin, canagliflozin, and empagliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/02.pdf. Accessed 9 July 2020.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Donnan JR, Grandy CA, Chibrikov E, Marra CA, Aubrey-Bassler K, Johnston K, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022577.
Rampersad C, Kraut E, Whitlock RH, Komenda P, Woo V, Rigatto C, et al. Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. Am J Kidney Dis. 2020;76(4):471–91.
Fujita T. Signal detection of adverse drug reactions. Jpn J Pharmacoepidemiol. 2009;14(1):27–36 (in Japanese).
FDA. FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Accessed 9 July 2020.
PMDA. JADER. (In Japanese). https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html. Accessed 9 July 2020.
Uppsala Monitoring Centre. VigiBase. https://www.who-umc.org/vigibase/vigibase/. Accessed 20 Dec 2020.
EMA. EudraVigilance. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance. Accessed 20 Dec 2020.
Narukawa M. Expectations for pharmacoepidemiology studies on drug risk management. Regul Sci Med Prod. 2016;6:335–43 (In Japanese).
Hinomura Y, Tadenuma H, Uehara K, Hidaka T, Murakami T, Narukawa M. Comparison of the characteristics of data contained in the drug side effect database by Japanese and US regulators. Japanese Society for Pharmacoepidemiology, 19th Annual Scientific Meeting. November 16th and 17th, 2013. (In Japanese). https://www.japic.or.jp/service/information/pdf/FAERS_JADER_data_compare.pdf. Accessed 9 July 2020.
Nomura K, Takahashi K, Hinomura Y, Kawaguchi G, Matsushita Y, Marui H, et al. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des Dev Ther. 2015;9:3031–41.
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27(12):1108–13.
Katsuhara Y, Ogawa T. Acute renal failure, ketoacidosis, and urogenital tract infections with SGLT2 inhibitors: signal detection using a Japanese spontaneous reporting database. Clin Drug Investig. 2020;40(7):645–52.
Raschi E, Parisotto M, Forcesi E, La Placa M, Marchesini G, De Ponti F, et al. Adverse events with sodium-glucose co-transporter-2 inhibitors: a global analysis of international spontaneous reporting systems. Nutr Metab Cardiovasc Dis. 2017;27(12):1098–107.
Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531–4.
MedDRA. About MedDRA. https://www.meddra.org/how-to-use/support-documentation/english/welcome. Accessed 20 Dec 2020.
Tsimihodimos V, Filippas-Ntekouan S, Elisaf M. SGLT1 inhibition: pros and cons. Eur J Pharmacol. 2018;5(838):153–6.
Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213–25.
Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: a systematic review and meta-analysis. PLoS ONE. 2020;15(6):e0234065.
Potier L, Roussel R, Velho G, Saulnier PJ, Bumbu A, Matar O, et al. Lower limb events in individuals with type 2 diabetes: evidence for an increased risk associated with diuretic use. Diabetologia. 2019;62(6):939–47.
Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018;20(3):740–4.
FDA. Highlights of prescribing information invokana (Canagliflozin). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204042s027lbl.pdf. Accessed 20 Dec 2020.
Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res. 2018;43(3):768–79.
Ishida T, Oh A, Hiroi S, Shimasaki Y, Tsuchihashi T. Current use of antihypertensive drugs in Japanese patients with hypertension: analysis by age group. Geriatr Gerontol Int. 2018;18(6):899–906.
Gu A, Farzadeh SN, Chang YJ, Kwong A, Lam S. Patterns of antihypertensive drug utilization among US adults with diabetes and comorbid hypertension: the National Health and Nutrition Examination Survey 1999–2014. Clin Med Insights Cardiol. 2019;13:1179546819839418.
Yamazaki T. A new topic for renin–angiotensin system inhibitors angiotensin converting enzyme inhibitor. Angiol Front. 2011;10(1):19–23.
McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332(7551):1177–81.
Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41(3):239–52.
Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing acute kidney injury reports among antibiotics: a pharmacovigilance study of the FDA adverse event reporting system (FAERS). Drug Saf. 2020;43(1):17–22.
Fan Q, Ma J, Zhang B, Li Q, Liu F, Zhao B. Assessment of acute kidney injury related to small-molecule protein kinase inhibitors using the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2020;86(5):655–62.
Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug administration adverse event reporting system database. Pharmacotherapy. 2018;38(8):785–93.
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases the notoriety bias. Drug Saf. 2007;30(10):891–8.
Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 2013;50(4):569–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this study.
Conflict of interest
Yukari Katsuhara is an employee of Takeda Pharmaceutical Company Limited and had been on leave during the research period. Shunya Ikeda has no conflict of interest.
Ethics approval
This study used anonymized information from the database, which is open to the public; therefore, in accordance with the 1964 Helsinki Declaration (and its amendments), institutional ethics approval was not required.
Consent to participate
This study used anonymized information from the database, which is open to the public; therefore, in accordance with the 1964 Helsinki Declaration (and its amendments), institutional ethics approval was not required.
Consent for publication
Not applicable.
Availability of data and material
The datasets analyzed in this study are available in the FDA Adverse Event Reporting System (FAERS): https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files
Code availability
Not applicable.
Authors contributions
Both authors were investigators in the study and participated in the study design, interpretation of the study results, and in the drafting, critical revision, and approval of the final version of the manuscript.
Acknowledgements
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katsuhara, Y., Ikeda, S. Correlations Between SGLT-2 Inhibitors and Acute Renal Failure by Signal Detection Using FAERS: Stratified Analysis for Reporting Country and Concomitant Drugs. Clin Drug Investig 41, 235–243 (2021). https://doi.org/10.1007/s40261-021-01006-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01006-9