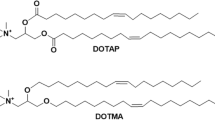
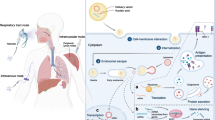
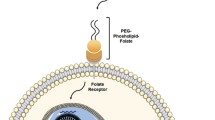
Abstract
Gene therapy for many diseases is rapidly becoming a reality, as demonstrated by the recent approval of various nucleic acid-based therapeutics. Non-viral systems such as lipid-based carriers, lipid nanoparticles (LNPs), for delivering different payloads including small interfering RNA, plasmid DNA, and messenger RNA have been particularly extensively explored and developed for clinical uses. One of the most important issues in LNP development is delivery to extrahepatic tissues. To achieve this, various lipids and lipid-like materials are being examined and screened. Several LNP formulations that target extrahepatic tissues, such as the spleen and the lungs have been developed by adjusting the lipid compositions of LNPs. However, mechanistic details of how the characteristics of LNPs affect delivery efficiency remains unclear. The purpose of this review is to provide an overview of LNP-based nucleic acid delivery focusing on LNP components and their structures, as well as discussing biological factors, such as biomolecular corona and cellular responses related to the delivery efficiency.






Similar content being viewed by others
References
van Dijk EL, et al. The third revolution in sequencing technology. Trends Genet. 2018;34(9):666–81.
Li K, et al. Bioinformatics approaches for anti-cancer drug discovery. Curr Drug Targets. 2020;21(1):3–17.
Sun YV, Hu YJ. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv Genet. 2016;93:147–90.
Joshi A, et al. Systems biology in cardiovascular disease: a multiomics approach. Nat Rev Cardiol. 2021;18(5):313–30.
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83.
Wang M, et al. Transformative network modeling of multi-omics data reveals detailed circuits, key regulators, and potential therapeutics for Alzheimer’s disease. Neuron. 2021;109(2):257-272.e14.
Baysoy A, et al. The technological landscape and applications of single-cell multi-omics. Nat Rev Mol Cell Biol. 2023;24:1–19.
SL G et al. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018; 20(5).
Piguet F, Alves S, Cartier N. Clinical gene therapy for neurodegenerative diseases: past, present, and future. Hum Gene Ther. 2017;28(11):988–1003.
Dunbar CE, et al. Gene therapy comes of age. Science. 2018;359(6372):eaan4672.
Gillmore JD, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493–502.
Uddin F, Rudin CM, Sen T. CRISPR gene therapy: applications, limitations, and implications for the future. Front Oncol. 2020;10:1387.
Ma L. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines. 2019;7(2):37.
Shigematsu H, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17(9):1152–61.
Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20(11):817–38.
Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2021;35:8971900211009650.
Chiriboga CA. Nusinersen for the treatment of spinal muscular atrophy. Expert Rev Neurother. 2017;17(10):955–62.
Raal FJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006.
Hoy SM. Patisiran: first global approval. Drugs. 2018;78(15):1625–31.
Cullis PR. Hope MJ, Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467.
Eygeris Y, et al. Chemistry of lipid nanoparticles for rna delivery. Acc Chem Res. 2022;55(1):2–12.
Khalil IA, et al. Lipid nanoparticles for cell-specific in vivo targeted delivery of nucleic acids. Biol Pharm Bull. 2020;43(4):584–95.
Li M, et al. The nano delivery systems and applications of mRNA. Eur J Med Chem. 2022;227: 113910.
Tenchov R, et al. Lipid nanoparticles-from liposomes to mrna vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982.
Herrera VL, et al. Nucleic acid nanomedicines in Phase II/III clinical trials: translation of nucleic acid therapies for reprogramming cells. Nanomedicine (Lond). 2018;13(16):2083–98.
Loughrey D, Dahlman JE. Non-liver mRNA delivery. Acc Chem Res. 2021;55:13.
Nakamura T, et al. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. Adv Drug Deliv Rev. 2022;188: 114417.
Cheng Q, et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313–20.
LoPresti ST, et al. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilities targeted mRNA delivery to the spleen and lungs. J Control Rel. 2022;345:819.
Liu S, et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20:5.
Dahlman JE, et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci USA. 2017;114(8):2060–5.
Paunovska K, et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 2018;18(3):2148–57.
Sago CD, et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc Natl Acad Sci USA. 2018;115(42):E9944-e9952.
Cui L, et al. Mechanistic studies of an automated lipid nanoparticle reveal critical pharmaceutical properties associated with enhanced mRNA functional delivery in vitro and in vivo. Small. 2022;18(9): e2105832.
Young RE, et al. Lipid nanoparticle composition drives mRNA delivery to the placenta. bioRxiv. 2022;20:534.
Kimura S, Harashima H. On the mechanism of tissue-selective gene delivery by lipid nanoparticles. J Control Rel. 2023;362:797.
Akinc A, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–64.
Fenton OS, et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv Mater. 2017;29(33):1606944.
Kimura S, et al. Novel lipid combination for delivery of plasmid DNA to immune cells in the spleen. J Control Rel. 2021;330:753–64.
Algarni A, et al. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci. 2022;10:2940.
Di J, et al. Biodistribution and non-linear gene expression of mRNA LNPs Affected by delivery route and particle size. Pharm Res. 2022;39(1):105–14.
Gilleron J, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638–46.
Gaurav S, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653.
Wang H, et al. The Niemann-pick C1 inhibitor NP3.47 enhances gene silencing potency of lipid nanoparticles containing siRNA. Mol Ther. 2016;24(12):2100–8.
Rennick JJ, Johnston APR, Parton RG. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16(3):266–76.
Delehedde C, et al. Intracellular routing and recognition of lipid-based mRNA nanoparticles. Pharmaceutics. 2021;13(7):945.
Lokugamage MP, et al. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv Mater. 2020;32(1): e1904905.
Siddarth P, et al. Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 2017;17(9):5711.
Paunovska K, et al. Increased PIP3 activity blocks nanoparticle mRNA delivery. Sci Adv. 2020;6(30):eaba5672.
Hatit MZC, et al. Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat Nanotechnol. 2022;17:310.
Dobrowolski C, et al. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat Nanotechnol. 2022;17:871.
Zhang Y, et al. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121(20):12181–277.
Kulkarni JA, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16:630.
Weng Y, et al. The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv. 2020;40: 107534.
Paunovska K, Loughrey D, Dahlman JE. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23:1–16.
Jayaraman M, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51(34):8529–33.
Sato Y, et al. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J Control Rel. 2019;295:140–52.
Dong Y, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci USA. 2014;111(11):3955–60.
Hassett KJ, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucl Acids. 2019;15:1–11.
Kulkarni JA, et al. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomedicine. 2017;13(4):1377–87.
Carrasco MJ, et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun Biol. 2021;4(1):956.
Kaczmarek JC, et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275: 120966.
Kauffman KJ, et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–6.
Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–10.
Speicher T, et al. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun. 2014;5:3862.
Love KT, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(5):1864–9.
Sun D, Lu ZR. Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharm Res. 2023;107:1–20.
Jörgensen AM, Wibel R, Bernkop-Schnürch A. Biodegradable cationic and ionizable cationic lipids: a roadmap for safer pharmaceutical excipients. Small. 2023;19(17): e2206968.
Whitehead KA, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277.
Fenton OS, et al. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Adv Mater. 2016;28(15):2939–43.
Qiu M, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci USA. 2021;118(10):e2020401118.
Sabnis S, et al. A Novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26(6):1509–19.
Akita H. Development of an SS-cleavable pH-activated lipid-like material (ssPalm) as a nucleic acid delivery device. Biol Pharm Bull. 2020;43(11):1617–25.
Zhu Y, et al. Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression. Nat Commun. 2022;13(1):4282.
Chander N, et al. Lipid nanoparticle mRNA systems containing high levels of sphingomyelin engender higher protein expression in hepatic and extra-hepatic tissues. Mol Ther Methods Clin Dev. 2023;30:235–45.
Sato Y, et al. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J Control Rel. 2012;163(3):267–76.
Kimura S, et al. Spleen selective enhancement of transfection activities of plasmid DNA driven by octaarginine and an ionizable lipid and its implications for cancer immunization. J Control Rel. 2019;313:70.
Lokugamage MP, et al. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv Mater (Deerfield Beach, Fla). 2020;32(1):1904905.
Sasaki K, et al. mRNA-loaded lipid nanoparticles targeting dendritic cells for cancer immunotherapy. Pharmaceutics. 2022;14(8):1572.
Zhang R, et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater Sci. 2021;9(4):1449–63.
Tanaka H, et al. Development of lipid-like materials for RNA delivery based on intracellular environment-responsive membrane destabilization and spontaneous collapse. Adv Drug Deliv Rev. 2020;154–155:210–26.
Shimosakai R, et al. mRNA-loaded lipid nanoparticles targeting immune cells in the spleen for use as cancer vaccines. Pharmaceuticals (Basel). 2022;15(8):1017.
Tenchov R, Sasso JM, Zhou QA. PEGylated lipid nanoparticle formulations: immunological safety and efficiency perspective. Bioconjug Chem. 2023;34(6):941–60.
Yang Q, et al. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol Pharm. 2014;11(4):1250–8.
Li M, et al. Brush conformation of polyethylene glycol determines the stealth effect of nanocarriers in the low protein adsorption regime. Nano Lett. 2021;21(4):1591–8.
Ja K, et al. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11(45):21733.
Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63(3):152–60.
Hatakeyama H, Akita H, Harashima H. The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull. 2013;36(6):892–9.
Mui BL, et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol Ther Nucl Acids. 2013;2: e139.
Hoang Thi TT, et al. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers (Basel). 2020;12(2):298.
Liu M, et al. A preliminary study of the innate immune memory of Kupffer cells induced by PEGylated nanoemulsions. J Control Rel. 2022;343:657–71.
Kierstead PH, et al. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J Control Rel. 2015;213:1–9.
Alberg I, et al. Polymeric nanoparticles with neglectable protein corona. Small. 2020;16(18): e1907574.
Suzuki T, et al. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: faster PEG shedding attenuates anti-PEG IgM production. Int J Pharm. 2020;588: 119792.
Krause MR, Regen SL. The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc Chem Res. 2014;47(12):3512–21.
Arakane K, Hayashi K. Influence of phospholipids purity and polyols on the temperature dependence of the permeability of liposomes. 日本化粧品技術者会誌. 1991;25(3):171.
Wang X, Quinn PJ. Cubic phase is induced by cholesterol in the dispersion of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine. Biochim Biophys Acta. 2002;1564(1):66–72.
Kimei H, et al. Alkyl chain length dependency in hydrolysis of liposomal phosphatidylcholine by dialkylphosphate. Chem Pharm Bull. 1995;43(10):1751.
Eygeris Y, et al. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 2020;20(6):4543–9.
Patel S, et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020;11(1):983.
Paunovska K, et al. Analyzing 2000 in vivo drug delivery data points reveals cholesterol structure impacts nanoparticle delivery. ACS Nano. 2018;12(8):8341–9.
Kalina P, et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv Mater (Deerfield Beach, Fla). 2019;31(14):1807748.
Álvarez-Benedicto E, et al. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater Sci. 2022;10(2):549–59.
Kulkarni JA, et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano. 2018;12(5):4787–95.
Li Z, et al. Acidification-induced structure evolution of lipid nanoparticles correlates with their in vitro gene transfections. ACS Nano. 2023;17:979–90.
Kulkarni JA, et al. Fusion-dependent formation of lipid nanoparticles containing macromolecular payloads. Nanoscale. 2019;11(18):9023–31.
Ramezanpour M, et al. Ionizable amino lipid interactions with POPC: implications for lipid nanoparticle function. Nanoscale. 2019;11(30):14141–6.
Yanez Arteta M, et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci USA. 2018;115(15):E3351-e3360.
Sebastiani F, et al. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021;15(4):6709–22.
Ermilova I, Swenson J. DOPC versus DOPE as a helper lipid for gene-therapies: molecular dynamics simulations with DLin-MC3-DMA. Phys Chem Chem Phys. 2020;22(48):28256–68.
Geng L, et al. Influence of lipid composition of messenger RNA-loaded lipid nanoparticles on the protein expression via intratracheal administration in mice. Int J Pharm. 2023;637: 122896.
Medjmedj A, et al. In cellulo and in vivo comparison of cholesterol, beta-sitosterol and dioleylphosphatidylethanolamine for lipid nanoparticle formulation of mRNA. Nanomaterials (Basel). 2022;12(14):2446.
Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona. Trends Biotechnol. 2017;35(3):257–64.
Caracciolo G, et al. Lipid composition: a “key factor” for the rational manipulation of the liposome-protein corona by liposome design. RSC Adv. 2015;5(8):5967–75.
Lokugamage MP, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Engl. 2021;5(9):1059–68.
Kim J, et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano. 2022;16(9):14792–806.
Melamed JR, et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 2023;9(4):eade1444.
Münter R, et al. Studying how administration route and dose regulates antibody generation against LNPs for mRNA delivery with single-particle resolution. Mol Ther Methods Clin Dev. 2023;29:450–9.
Francia V, et al. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug Chem. 2020;31:2046.
Palmieri V, Caracciolo G. Tuning the immune system by nanoparticle-biomolecular corona. Nanoscale Adv. 2022;4(16):3300–8.
Monopoli MP, et al. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–86.
Schottler S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372–7.
Papini E, Tavano R, Mancin F. Opsonins and dysopsonins of nanoparticles: facts, concepts, and methodological guidelines. Front Immunol. 2020;11: 567365.
Giulimondi F, et al. Opsonin-deficient nucleoproteic corona endows unPEGylated liposomes with stealth properties in vivo. ACS Nano. 2022;16(2):2088–100.
Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–43.
Tonigold M, et al. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat Nanotechnol. 2018;13(9):862–9.
Albert C, et al. Monobody adapter for functional antibody display on nanoparticles for adaptable targeted delivery applications. Nat Commun. 2022;13(1):5998.
Sivaram AJ, et al. Recent advances in the generation of antibody-nanomaterial conjugates. Adv Healthc Mater. 2018;7(1):1700607.
Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci USA. 2021;118(52):e2109256118.
Qiu M, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2022;119(8):e2116271119.
Melamed JR, et al. Lipid nanoparticle chemistry determines how nucleoside base modifications alter mRNA delivery. J Control Release. 2022;341:206–14.
Kowalski PS, et al. Ionizable amino-polyesters synthesized via ring opening polymerization of tertiary amino-alcohols for tissue selective mRNA delivery. Adv Mater. 2018;30:e1801151.
Digiacomo L, et al. An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles. Nanoscale. 2017;9(44):17254–62.
Cai R, et al. Dynamic intracellular exchange of nanomaterials’ protein corona perturbs proteostasis and remodels cell metabolism. Proc Natl Acad Sci USA. 2022;119(23): e2200363119.
Sago CD, et al. Modifying a commonly expressed endocytic receptor retargets nanoparticles in vivo. Nano Lett. 2018;18(12):7590–600.
Patel S, et al. Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 2017;17(9):5711–8.
Lokugamage MP, et al. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv Mater. 2020;32(1):1904905.
Radmand A, et al. The transcriptional response to lung-targeting lipid nanoparticles in vivo. Nano Lett. 2023;23:993.
Hatit MZC, et al. Nanoparticle stereochemistry-dependent endocytic processing improves in vivo mRNA delivery. Nat Chem. 2023;15(4):508–15.
Davidsson M, et al. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proc Natl Acad Sci USA. 2019;116(52):27053–62.
Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34(2):204–9.
Goertsen D, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci. 2022;25(1):106–15.
Ravindra Kumar S, et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat Methods. 2020;17(5):541–50.
Rhym LH, et al. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat Biomed Eng. 2023;7:901.
Kimura S, Harashima H. Non-invasive gene delivery across the blood-brain barrier: present and future perspectives. Neural Regen Res. 2022;17(4):785–7.
Kim J, et al. High-throughput evaluation of polymeric nanoparticles for tissue-targeted gene expression using barcoded plasmid DNA. J Control Rel. 2021;337:105.
Ho D, Wang P, Kee T. Artificial intelligence in nanomedicine. Nanoscale Horiz. 2019;4(2):365–77.
Acknowledgements
We wish to thank Dr. Milton Feather for his helpful advice in editing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received for this review.
Conflict of interest
The authors declare no competing financial interest.
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Seigo Kimura: writing—original draft. Hideyoshi Harashima: supervision. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kimura, S., Harashima, H. Nano–Bio Interactions: Exploring the Biological Behavior and the Fate of Lipid-Based Gene Delivery Systems. BioDrugs 38, 259–273 (2024). https://doi.org/10.1007/s40259-024-00647-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-024-00647-4