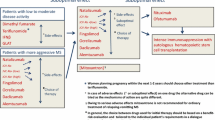
Abstract
The use of monoclonal antibodies in multiple sclerosis (MS) patients is in a transitional period. Studies regarding well-established, effective antibodies such as natalizumab and alemtuzumab focus more and more on long-term efficacy and safety, risk management, and treating complications. Primary progressive MS, a disease that was long considered to be unmodifiable, is currently in focus following ocrelizumab being approved as the first drug with a proven beneficial effect on the disease course. Conversely, post-marketing safety mechanisms have also proven to function as daclizumab has been suspended after a series of relevant serious adverse events. Currently, new therapies are emerging that promise more convenience and an improved safety profile (ofatumumab) or remyelinating potential with clinical improvement (opicinumab). While it is very unlikely that monoclonal antibodies will ever cure MS, they have become very valuable therapeutic tools to better patient outcomes. This review focuses on developments of monoclonal antibodies used in the past, present, and near future in MS patients.
Similar content being viewed by others
References
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–36. https://doi.org/10.1016/S0140-6736(18)30481-1.
Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. https://doi.org/10.1159/000367614.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–86. https://doi.org/10.1212/WNL.0000000000000560.
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:789–800. https://doi.org/10.1212/WNL.0000000000005345.
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–88. https://doi.org/10.1212/WNL.0000000000005347.
Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203. https://doi.org/10.1080/19420862.2018.1415671.
Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. https://doi.org/10.1136/bmj.i3518.
Soelberg Sorensen P. Safety concerns and risk management of multiple sclerosis therapies. Acta Neurol Scand. 2017;136:168–86. https://doi.org/10.1111/ane.12712.
Winkelmann A, Loebermann M, Reisinger EC, Hartung H-P, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12:217–33. https://doi.org/10.1038/nrneurol.2016.21.
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GPA, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. https://doi.org/10.1056/NEJMoa020696.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. https://doi.org/10.1056/NEJMoa044397.
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–60. https://doi.org/10.1016/S1474-4422(09)70021-3.
Oshima Y, Tanimoto T, Yuji K, Tojo A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. 2018. https://doi.org/10.1177/1352458518786075.
Biogen. Biogen internal data. Safety update on natalizumab; September 2018. www.tysabri.de. Accessed 10 Dec 2018.
Butzkueven H, Kappos L, Pellegrini F, Trojano M, Wiendl H, Patel RN, et al. Efficacy and safety of natalizumab in multiple sclerosis: Interim observational programme results. J Neurol Neurosurg Psychiatry. 2014;85:1190–7. https://doi.org/10.1136/jnnp-2013-306936.
Lorefice L, Fenu G, Gerevini S, Frau J, Coghe G, Barracciu MA, et al. PML in a person with multiple sclerosis: is teriflunomide the felon? Neurology. 2018;90:83–5. https://doi.org/10.1212/WNL.0000000000004804.
Bauer J, Gold R, Adams O, Lassmann H. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome (IRIS). Acta Neuropathol. 2015;130:751–64. https://doi.org/10.1007/s00401-015-1471-7.
Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17:467–80. https://doi.org/10.1016/S1474-4422(18)30040-1.
Ho P-R, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925–33. https://doi.org/10.1016/S1474-4422(17)30282-X.
Warnke C, Ramanujam R, Plavina T, Bergström T, Goelz S, Subramanyam M, et al. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry. 2013;84:1199–205. https://doi.org/10.1136/jnnp-2012-304332.
Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–12. https://doi.org/10.1002/ana.24286.
Plavina T, Muralidharan KK, Kuesters G, Mikol D, Evans K, Subramanyam M, et al. Reversibility of the effects of natalizumab on peripheral immune cell dynamics in MS patients. Neurology. 2017;89:1584–93. https://doi.org/10.1212/WNL.0000000000004485.
Bianco A, Rossini PM, Mirabella M. Moving to fingolimod from natalizumab in multiple sclerosis: the ENIGM is not solved. JAMA Neurol. 2014;71:924–5. https://doi.org/10.1001/jamaneurol.2014.1135.
Derfuss T, Kovarik JM, Kappos L, Savelieva M, Chhabra R, Thakur A, et al. α4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol Neuroimmunol Neuroinflamm. 2017;4:e388. https://doi.org/10.1212/NXI.0000000000000388.
Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–8. https://doi.org/10.1212/WNL.0b013e31828c2fa1.
Wijburg MT, Warnke C, Barkhof F, Uitdehaag BMJ, Killestein J, Wattjes MP. Performance of PML diagnostic criteria in natalizumab-associated PML: data from the Dutch-Belgian cohort. J Neurol Neurosurg Psychiatry. 2018. https://doi.org/10.1136/jnnp-2018-318261.
Peters J, Williamson E. Natalizumab therapy is associated with changes in serum JC virus antibody indices over time. J Neurol. 2017;264:2409–12. https://doi.org/10.1007/s00415-017-8643-4.
Koolaji S, Allahabadi NS, Ahmadi A, Eskandarieh S, Moghadasi AN, Azimi AR, et al. Anti-JC virus antibody sera positivity and index value among patients with multiple sclerosis may be correlated with age, sex, and area of residence. J Neurovirol. 2018;24(5):570–6. https://doi.org/10.1007/s13365-018-0646-0.
van Kempen Z, Leurs CE, Vennegoor A, Wattjes MP, Rispens T, Uitdehaag BM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy is not preceded by elevated drug concentrations. Mult Scler. 2017;23:995–9. https://doi.org/10.1177/1352458516684023.
Fissolo N, Pignolet B, Matute-Blanch C, Triviño JC, Miró B, Mota M, et al. Matrix metalloproteinase 9 is decreased in natalizumab-treated multiple sclerosis patients at risk for progressive multifocal leukoencephalopathy. Ann Neurol. 2017;82:186–95. https://doi.org/10.1002/ana.24987.
Antoniol C, Stankoff B. Immunological markers for PML prediction in MS patients treated with natalizumab. Front Immunol. 2014;5:668. https://doi.org/10.3389/fimmu.2014.00668.
Schwab N, Schneider-Hohendorf T, Posevitz V, Breuer J, Göbel K, Windhagen S, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–71. https://doi.org/10.1212/WNL.0b013e3182a351fb.
Lieberman LA, Zeng W, Singh C, Wang W, Otipoby KL, Loh C, et al. CD62L is not a reliable biomarker for predicting PML risk in natalizumab-treated R-MS patients. Neurology. 2016;86:375–81. https://doi.org/10.1212/WNL.0000000000002314.
Scarpazza C, Prosperini L, De Rossi N, Moiola L, Sormani MP, Gerevini S, Capra R. To do or not to do? Plasma exchange and timing of steroid administration in progressive multifocal leukoencephalopathy. Ann Neurol. 2017;82:697–705. https://doi.org/10.1002/ana.25070.
Landi D, de Rossi N, Zagaglia S, Scarpazza C, Prosperini L, Albanese M, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology. 2017;88:1144–52. https://doi.org/10.1212/WNL.0000000000003740.
Yamout BI, Sahraian MA, Ayoubi NE, Tamim H, Nicolas J, Khoury SJ, et al. Efficacy and safety of natalizumab extended interval dosing. Mult Scler Relat Disord. 2018;24:113–6. https://doi.org/10.1016/j.msard.2018.06.015.
Kapoor R, Ho P-R, Campbell N, Chang I, Deykin A, Forrestal F, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17:405–15. https://doi.org/10.1016/S1474-4422(18)30069-3.
Zhang X, Tao Y, Chopra M, Ahn M, Marcus KL, Choudhary N, et al. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191:5867–74. https://doi.org/10.4049/jimmunol.1301926.
Hartung H-P, Aktas O, Boyko AN. Alemtuzumab: a new therapy for active relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:22–34. https://doi.org/10.1177/1352458514549398.
Coles AJ, Compston DAS, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. https://doi.org/10.1056/NEJMoa0802670.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28. https://doi.org/10.1016/S0140-6736(12)61769-3.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–39. https://doi.org/10.1016/S0140-6736(12)61768-1.
Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol. 2011;10:338–48. https://doi.org/10.1016/S1474-4422(11)70020-5.
Hartung H-P, Aktas O. Evolution of multiple sclerosis treatment: next generation therapies meet next generation efficacy criteria. Lancet Neurol. 2011;10:293–5. https://doi.org/10.1016/S1474-4422(11)70043-6.
Havrdova E, Arnold DL, Cohen JA, Hartung H-P, Fox EJ, Giovannoni G, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89:1107–16. https://doi.org/10.1212/WNL.0000000000004313.
Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung H-P, Havrdova E, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017;89:1117–26. https://doi.org/10.1212/WNL.0000000000004354.
Giovannoni G, Cohen JA, Coles AJ, Hartung H-P, Havrdova E, Selmaj KW, et al. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology. 2016;87:1985–92. https://doi.org/10.1212/WNL.0000000000003319.
Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16:271–81. https://doi.org/10.1016/S1474-4422(17)30007-8.
Lizak N, Lugaresi A, Alroughani R, Lechner-Scott J, Slee M, Havrdova E, et al. Highly active immunomodulatory therapy ameliorates accumulation of disability in moderately advanced and advanced multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:196–203. https://doi.org/10.1136/jnnp-2016-313976.
Zimmermann M, Brouwer E, Tice JA, Seidner M, Loos AM, Liu S, et al. Disease-modifying therapies for relapsing-remitting and primary progressive multiple sclerosis: a cost-utility analysis. CNS Drugs. 2018. https://doi.org/10.1007/s40263-018-0566-9.
Haghikia A, Dendrou CA, Schneider R, Grüter T, Postert T, Matzke M, et al. Severe B-cell-mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol. 2017;16:104–6. https://doi.org/10.1016/S1474-4422(16)30382-9.
Wehrum T, Beume L-A, Stich O, Mader I, Mäurer M, Czaplinski A, et al. Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology. 2018;90:e601–5. https://doi.org/10.1212/WNL.0000000000004950.
Barton J, Hardy TA, Riminton S, Reddel SW, Barnett Y, Coles A, Barnett MH. Tumefactive demyelination following treatment for relapsing multiple sclerosis with alemtuzumab. Neurology. 2017;88:1004–6. https://doi.org/10.1212/WNL.0000000000003694.
Pfeuffer S, Beuker C, Ruck T, Lenze F, Wiendl H, Melzer N, Meuth SG. Acute cholecystitis during treatment with alemtuzumab in 3 patients with RRMS. Neurology. 2016;87:2380–1. https://doi.org/10.1212/WNL.0000000000003379.
Sauer E-M, Schliep S, Manger B, Lee D-H, Linker RA. Microscopic polyangiitis after alemtuzumab treatment in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e488. https://doi.org/10.1212/NXI.0000000000000488.
Graf J, Ringelstein M, Lepka K, Schaller J, Quack H, Hartung H-P, et al. Acute sarcoidosis in a multiple sclerosis patient after alemtuzumab treatment. Mult Scler. 2018;24(13):1776–8. https://doi.org/10.1177/1352458518771276.
Willis MD, Hope-Gill B, Flood-Page P, Joseph F, Needham E, Jones J, et al. Sarcoidosis following alemtuzumab treatment for multiple sclerosis. Mult Scler. 2018;24(13):1779–82. https://doi.org/10.1177/1352458518790391.
Pfeuffer S. Sarcoidosis following alemtuzumab treatment: autoimmunity mediated by T cells and interferon-γ. Mult Scler. 2018;24(13):1783–4. https://doi.org/10.1177/1352458518804124.
Leussink VI, Reifenberger J, Hartung H-P. Case of alopecia universalis associated with alemtuzumab treatment in MS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e454. https://doi.org/10.1212/NXI.0000000000000454.
Ruck T, Pfeuffer S, Schulte-Mecklenbeck A, Gross CC, Lindner M, Metze D, et al. Vitiligo after alemtuzumab treatment: secondary autoimmunity is not all about B cells. Neurology. 2018. https://doi.org/10.1212/WNL.0000000000006648.
Rau D, Lang M, Harth A, Naumann M, Weber F, Tumani H, et al. Listeria meningitis complicating alemtuzumab treatment in multiple sclerosis—report of two cases. Int J Mol Sci. 2015;16:14669–76. https://doi.org/10.3390/ijms160714669.
Canham LJW, Manara A, Fawcett J, Rolinski M, Mortimer A, Inglis KEA, et al. Mortality from Listeria monocytogenes meningoencephalitis following escalation to alemtuzumab therapy for relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2018;24:38–41. https://doi.org/10.1016/j.msard.2018.05.014.
Meunier B, Rico A, Seguier J, Boutiere C, Ebbo M, Harle JR, et al. Life-threatening autoimmune warm hemolytic anemia following treatment for multiple sclerosis with alemtuzumab. Mult Scler. 2018;24:811–3. https://doi.org/10.1177/1352458517729766.
Saarela M, Senthil K, Jones J, Tienari PJ, Soilu-Hänninen M, Airas L, et al. Hemophagocytic lymphohistiocytosis in 2 patients with multiple sclerosis treated with alemtuzumab. Neurology. 2018;90:849–51. https://doi.org/10.1212/WNL.0000000000005420.
Brownlee WJ, Chataway J. Opportunistic infections after alemtuzumab: new cases of norcardial infection and cytomegalovirus syndrome. Mult Scler. 2017;23:876–7. https://doi.org/10.1177/1352458517693440.
Clerico M, de Mercanti S, Artusi CA, Durelli L, Naismith RT. Active CMV infection in two patients with multiple sclerosis treated with alemtuzumab. Mult Scler. 2017;23:874–6. https://doi.org/10.1177/1352458516688350.
Blasco MR, Ramos A, Malo CG, García-Merino A. Acute pneumonitis and pericarditis related to alemtuzumab therapy in relapsing-remitting multiple sclerosis. J Neurol. 2017;264:168–9. https://doi.org/10.1007/s00415-016-8306-x.
Wray S, Havrdova E, Snydman DR, Arnold DL, Cohen JA, Coles AJ, et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler. 2018. https://doi.org/10.1177/1352458518796675.
Pariani N, Willis M, Muller I, Healy S, Nasser T, McGowan A, et al. Alemtuzumab-induced thyroid dysfunction exhibits distinctive clinical and immunological features. J Clin Endocrinol Metab. 2018;103:3010–8. https://doi.org/10.1210/jc.2018-00359.
Steinman L. Induction of new autoimmune diseases after alemtuzumab therapy for multiple sclerosis: learning from adversity. JAMA Neurol. 2017;74:907–8. https://doi.org/10.1001/jamaneurol.2017.0325.
Graf J, Leussink VI, Dehmel T, Ringelstein M, Goebels N, Adams O, et al. Infectious risk stratification in multiple sclerosis patients receiving immunotherapy. Ann Clin Transl Neurol. 2017;4:909–14. https://doi.org/10.1002/acn3.491.
Guarnera C, Bramanti P, Mazzon E. Alemtuzumab: a review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther Clin Risk Manag. 2017;13:871–9. https://doi.org/10.2147/TCRM.S134398.
Bielekova B. Daclizumab therapy for multiple sclerosis. Neurotherapeutics. 2013;10:55–67. https://doi.org/10.1007/s13311-012-0147-4.
Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373:1418–28. https://doi.org/10.1056/NEJMoa1501481.
Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue E-W, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–75. https://doi.org/10.1016/S0140-6736(12)62190-4.
Kappos L, Havrdova E, Giovannoni G, Khatri BO, Gauthier SA, Greenberg SJ, et al. No evidence of disease activity in patients receiving daclizumab versus intramuscular interferon beta-1a for relapsing-remitting multiple sclerosis in the DECIDE study. Mult Scler. 2017;23:1736–47. https://doi.org/10.1177/1352458516683266.
Benedict RH, Cohan S, Lynch SG, Riester K, Wang P, Castro-Borrero W, et al. Improved cognitive outcomes in patients with relapsing-remitting multiple sclerosis treated with daclizumab beta: results from the DECIDE study. Mult Scler. 2018;24:795–804. https://doi.org/10.1177/1352458517707345.
Lancet T. End of the road for daclizumab in multiple sclerosis. Lancet. 2018;391:1000. https://doi.org/10.1016/S0140-6736(18)30565-8.
Devlin M, Swayne A, Newman M, O’Gorman C, Brown H, Ong B, et al. A case of immune-mediated encephalitis related to daclizumab therapy. Mult Scler. 2018. https://doi.org/10.1177/1352458518792403.
Williams T, Chataway J. Immune-mediated encephalitis with daclizumab: the final nail. Mult Scler. 2018. https://doi.org/10.1177/1352458518791374.
European Medical Agency, Pharmacovigilance Risk Assessment Committee (PRAC). Assessment report on provisional measures, Procedure under Article 20 of Regulation (EC) No 726/2004 resulting from pharmacovigilance data pharmacovigilance data Zinbryta, Procedure number: EMEA/H/A-20/1462/C/003862/0018; 2018. https://www.ema.europa.eu/documents/referral/zinbryta-article-20-referral-prac-assessment-report_en.pdf. Accessed 20 Aug 2018.
Luessi F, Engel S, Spreer A, Bittner S, Zipp F. GFAPα IgG-associated encephalitis upon daclizumab treatment of MS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e481. https://doi.org/10.1212/NXI.0000000000000481.
Krueger JG, Kircik L, Hougeir F, Friedman A, You X, Lucas N, et al. Cutaneous adverse events in the randomized, double-blind, active-comparator DECIDE study of daclizumab high-yield process versus intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis. Adv Ther. 2016;33:1231–45. https://doi.org/10.1007/s12325-016-0353-2.
Cortese I, Ohayon J, Fenton K, Lee C-C, Raffeld M, Cowen EW, et al. Cutaneous adverse events in multiple sclerosis patients treated with daclizumab. Neurology. 2016;86:847–55. https://doi.org/10.1212/WNL.0000000000002417.
Rauer S, Stork L, Urbach H, Stathi A, Marx A, Süß P, et al. Drug reaction with eosinophilia and systemic symptoms after daclizumab therapy. Neurology. 2018;91:e359–63. https://doi.org/10.1212/WNL.0000000000005854.
Franks SE, Getahun A, Hogarth PM, Cambier JC. Targeting B cells in treatment of autoimmunity. Curr Opin Immunol. 2016;43:39–45. https://doi.org/10.1016/j.coi.2016.09.003.
Schuh E, Berer K, Mulazzani M, Feil K, Meinl I, Lahm H, et al. Features of human CD3+CD20+ T cells. J Immunol. 2016;197:1111–7. https://doi.org/10.4049/jimmunol.1600089.
Palanichamy A, Jahn S, Nickles D, Derstine M, Abounasr A, Hauser SL, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol. 2014;193:580–6. https://doi.org/10.4049/jimmunol.1400118.
Wilk E, Witte T, Marquardt N, Horvath T, Kalippke K, Scholz K, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheumatol. 2009;60:3563–71. https://doi.org/10.1002/art.24998.
Stüve O, Cepok S, Elias B, Saleh A, Hartung H-P, Hemmer B, Kieseier BC. Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing-remitting multiple sclerosis. Arch Neurol. 2005;62:1620–3. https://doi.org/10.1001/archneur.62.10.1620.
Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons J-A. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. https://doi.org/10.1016/j.jneuroim.2006.06.029.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. https://doi.org/10.1056/NEJMoa0706383.
Alcalá C, Gascón F, Pérez-Miralles F, Gil-Perotín S, Navarré A, Boscá I, et al. Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol. 2018;265:1690–7. https://doi.org/10.1007/s00415-018-8899-3.
Scotti B, Disanto G, Sacco R, Guigli M, Zecca C, Gobbi C. Effectiveness and safety of rituximab in multiple sclerosis: an observational study from Southern Switzerland. PLoS One. 2018;13:e0197415. https://doi.org/10.1371/journal.pone.0197415.
Durozard P, Maarouf A, Boutiere C, Ruet A, Brochet B, Vukusic S, et al. Efficacy of rituximab in refractory RRMS. Mult Scler. 2018. https://doi.org/10.1177/1352458518772748.
Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, Piehl F. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75:320–7. https://doi.org/10.1001/jamaneurol.2017.4011.
Spelman T, Frisell T, Piehl F, Hillert J. Comparative effectiveness of rituximab relative to IFN-β or glatiramer acetate in relapsing-remitting MS from the Swedish MS registry. Mult Scler. 2018;24:1087–95. https://doi.org/10.1177/1352458517713668.
Memon AB, Javed A, Caon C, Srivastawa S, Bao F, Bernitsas E, et al. Long-term safety of rituximab induced peripheral B-cell depletion in autoimmune neurological diseases. PLoS One. 2018;13:e0190425. https://doi.org/10.1371/journal.pone.0190425.
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87:2074–81. https://doi.org/10.1212/WNL.0000000000003331.
Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71. https://doi.org/10.1002/ana.21867.
Hartung H-P, Aktas O. Bleak prospects for primary progressive multiple sclerosis therapy: downs and downs, but a glimmer of hope. Ann Neurol. 2009;66:429–32. https://doi.org/10.1002/ana.21880.
Mitka M. FDA: increased HBV reactivation risk with ofatumumab or rituximab. JAMA. 2013;310:1664. https://doi.org/10.1001/jama.2013.281115.
Buti M, Manzano ML, Morillas RM, García-Retortillo M, Martín L, Prieto M, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS One. 2017;12:e0184550. https://doi.org/10.1371/journal.pone.0184550.
Dunn N, Juto A, Ryner M, Manouchehrinia A, Piccoli L, Fink K, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler. 2018;24(9):1224–33. https://doi.org/10.1177/1352458517720044.
Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–40. https://doi.org/10.1182/blood-2008-10-186999.
Tallantyre EC, Whittam DH, Jolles S, Paling D, Constantinesecu C, Robertson NP, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. 2018;265:1115–22. https://doi.org/10.1007/s00415-018-8812-0.
Ram R, Ben-Bassat I, Shpilberg O, Polliack A, Raanani P. The late adverse events of rituximab therapy—rare but there! Leuk Lymphoma. 2009;50:1083–95. https://doi.org/10.1080/10428190902934944.
Rissanen E, Remes K, Airas L. Severe neutropenia after rituximab-treatment of multiple sclerosis. Mult Scler Relat Disord. 2018;20:3–5. https://doi.org/10.1016/j.msard.2017.12.005.
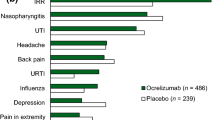
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–34. https://doi.org/10.1056/NEJMoa1601277.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–20. https://doi.org/10.1056/NEJMoa1606468.
Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–87. https://doi.org/10.1016/S0140-6736(11)61649-8.
Havrdová E, Arnold DL, Bar-Or A, Comi G, Hartung H-P, Kappos L, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018;4:2055217318760642. https://doi.org/10.1177/2055217318760642.
Hauser S. Safety of ocrelizumab in multiple sclerosis: updated analysis in patients with relapsing and primary progressive multiple sclerosis. Platform presentation number S36.001. AAN Annual Meeting; 2018. http://n.neurology.org/content/90/15_Supplement/S36.001. Accessed 20 Aug 2018.
Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82:573–81. https://doi.org/10.1212/WNL.0000000000000125.
Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90:e1805–14. https://doi.org/10.1212/WNL.0000000000005516.
Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–51. https://doi.org/10.1038/nn1460.
Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–15. https://doi.org/10.1002/ana.21581.
Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES, Yuan Q, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–33. https://doi.org/10.1038/nm1664.
Aktas O, Albrecht P, Hartung H-P. Optic neuritis as a phase 2 paradigm for neuroprotection therapies of multiple sclerosis: update on current trials and perspectives. Curr Opin Neurol. 2016;29:199–204. https://doi.org/10.1097/WCO.0000000000000327.
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–99. https://doi.org/10.1016/S1474-4422(16)30377-5.
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch LJ, et al. Predictors of response to opicinumab in acute optic neuritis. Ann Clin Transl Neurol. 2018;41:1017. https://doi.org/10.1002/acn3.620.
Ranger A, Ray S, Szak S, Dearth A, Allaire N, Murray R, et al. Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies. Neurol Neuroimmunol Neuroinflamm. 2018;5:e417. https://doi.org/10.1212/NXI.0000000000000417.
Kremer D, Göttle P, Hartung H-P, Küry P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci. 2016;39:246–63. https://doi.org/10.1016/j.tins.2016.02.004.
Moccia M, de Stefano N, Barkhof F. Imaging outcome measures for progressive multiple sclerosis trials. Mult Scler. 2017;23:1614–26. https://doi.org/10.1177/1352458517729456.
Šega-Jazbec S, Barun B, Horvat Ledinek A, Fabekovac V, Krbot Skorić M, Habek M. Management of infusion related reactions associated with alemtuzumab in patients with multiple sclerosis. Mult Scler Relat Disord. 2017;17:151–3. https://doi.org/10.1016/j.msard.2017.07.019.
Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016;22:837–50. https://doi.org/10.1212/CON.0000000000000328.
Voskuhl R, Momtazee C. Pregnancy: effect on multiple sclerosis, treatment considerations, and breastfeeding. Neurotherapeutics. 2017;14:974–84. https://doi.org/10.1007/s13311-017-0562-7.
Houtchens MK, Edwards NC, Phillips AL. Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology. 2018;91:e1570–8. https://doi.org/10.1212/WNL.0000000000006382.
European Medical Agency. Tysabri summary of product characteristics; 2018. https://ec.europa.eu/health/documents/community-register/2018/20180802142037/anx_142037_en.pdf. Accessed 20 Nov 2018.
European Medical Agency. Lemtrada summary of product characteristics; 2018. https://www.ema.europa.eu/documents/product-information/lemtrada-epar-product-information_en.pdf. Accessed 20 Nov 2018.
European Medical Agency. Ocrevus summary of product characteristics; 2018. https://www.ema.europa.eu/documents/product-information/ocrevus-epar-product-information_en.pdf. Accessed 20 Nov 2018.
Trojano M, Tintore M, Montalban X, Hillert J, Kalincik T, Iaffaldano P, et al. Treatment decisions in multiple sclerosis - insights from real-world observational studies. Nat Rev Neurol. 2017;13:105–18. https://doi.org/10.1038/nrneurol.2016.188.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflict of interest
Jonas Graf received travel/meeting/accommodation reimbursements from Biogen, and Merck Serono. Orhan Aktas received, with approval of the Rector of Heinrich-Heine-University, grants from the German Research Foundation (DFG) and the German Ministry for Education and Research (BMBF) as part of the German Competence Network Multiple Sclerosis (Kompetenznetz Multiple Sklerose (KKNMS); for NEMOS (Neuromyelitis optica Studiengruppe, German Neuromyelitis Optica Study group) NationNMO-PAT FKZ 01GI1602B), the Eugène Devic European Network (EU-FP7), and honoraria and travel/accommodation/meeting expenses from Almirall, Bayer, Biogen, Medimmune, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. Konrad Rejdak has no conflicts of interest to declare. Hans-Peter Hartung received, with approval of the Rector of Heinrich-Heine-University and the CEO of University of Düsseldorf Hospital, honoraria for consulting, serving on steering committees, and speaking from Biogen, CSL Behring, Geneuro, Genzyme, LFB, Medimmune, Merck, Novartis, Octapharma, Opexa, Receptos/Celgene, Roche, Sanofi, and Teva.
Rights and permissions
About this article
Cite this article
Graf, J., Aktas, O., Rejdak, K. et al. Monoclonal Antibodies for Multiple Sclerosis: An Update. BioDrugs 33, 61–78 (2019). https://doi.org/10.1007/s40259-018-0327-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0327-9