Abstract
Background
Studies have shown that centralising surgical treatment for some cancers can improve patient outcomes, but there is limited evidence of the impact on costs or health-related quality of life.
Objectives
We report the results of a cost-utility analysis of the RESPECT-21 study using difference-in-differences, which investigated the reconfiguration of specialist surgery services for four cancers in an area of London, compared to the Rest of England (ROE).
Methods
Electronic health records data were obtained from the National Cancer Registration and Analysis Service for patients diagnosed with one of the four cancers of interest between 2012 and 2017. The analysis for each tumour type used a short-term decision tree followed by a 10-year Markov model with 6-monthly cycles. Costs were calculated by applying National Health Service (NHS) Reference Costs to patient-level hospital resource use and supplemented with published data. Cancer-specific preference-based health-related quality-of-life values were obtained from the literature to calculate quality-adjusted life-years (QALYs). Total costs and QALYs were calculated before and after the reconfiguration, in the London Cancer (LC) area and in ROE, and probabilistic sensitivity analysis was performed to illustrate the uncertainty in the results.
Results
At a threshold of £30,000/QALY gained, LC reconfiguration of prostate cancer surgery services had a 79% probability of having been cost-effective compared to non-reconfigured services using difference-in-differences. The oesophago-gastric, bladder and renal reconfigurations had probabilities of 62%, 49% and 12%, respectively, of being cost-effective at the same threshold. Costs and QALYs per surgical patient increased over time for all cancers across both regions to varying degrees. Bladder cancer surgery had the smallest patient numbers and changes in costs, and QALYs were not significant. The largest improvement in outcomes was in renal cancer surgery in ROE, making the relative renal improvements in LC appear modest, and the probability of the LC reconfiguration having been cost-effective low.
Conclusions
Prostate cancer reconfigurations had the highest probability of being cost-effective. It is not clear, however, whether the prostate results can be considered in isolation, given the reconfigurations occurred simultaneously with other system changes, and healthcare delivery in the NHS is highly networked and collaborative. Routine collection of quality-of-life measures such as the EQ-5D-5L would have improved the analysis.
Similar content being viewed by others
This analysis suggested that the London Cancer region changes in specialist cancer surgery services were most cost-effective in prostate cancer, followed by oesophago-gastric and bladder cancer, and the changes in specialist renal cancer surgery were not cost-effective. |
The individual cancer pathways were, however, not reconfigured in isolation, and delivery of National Health Service (NHS) healthcare services is a highly networked and collaborative activity. The results of the four analyses should therefore be considered together as a group and not separately. |
Comprehensive routine collection of patient-level health-related quality-of-life information (EQ-5D-5L) would improve this type of observational analysis. |
1 Introduction
1.1 Background
Major system change in a healthcare context involves service reconfiguration or reorganisation at a regional level [1]. Service centralisations, leading to higher patient volumes at fewer sites, have improved outcomes for some cancers, for example, by reducing mortality and length of stay (LOS) in hospital [2, 3]. Improvements following centralisations are thought to be driven through improved quality of care and better trained staff arising from increased volumes [4,5,6,7]. Reconfiguration could also lead to cost reductions as well as clinical improvements [4, 8], for example, via economies of scale, where the newly centralised services deliver improved care at similar or reduced cost [9, 10]. If the cost of implementing the new system is not greater than any longer-term savings, and outcomes are the same or better, then the centralised model should be more cost-effective than the previous system [11, 12]. Economic evaluations of major system change though are challenging and therefore not commonly conducted [8, 13,14,15].
The ‘Reorganising specialist cancer surgery for the twenty-first century: a mixed methods evaluation’ (RESPECT-21) programme examined processes and impacts of service delivery changes taking place in specialist surgery services for prostate, bladder, renal and oesophago-gastric (OG) cancers in parts of London and Greater Manchester (GM), and considered how lessons learned could apply in future centralisations [16]. These cancers were selected as they were being centralised in both the London Cancer (LC) region and in GM, potentially permitting analysis of changes in different contexts. The planned reconfigurations were anticipated to affect process variables (e.g. waiting times) and clinical variables (e.g. improving surgeons’ and associated surgical and wider teams’ skills through specialisation), and in turn to improve patient outcomes (mortality, hospital LOS and rate of readmissions) [16]. Between 2012 and 2016, an integrated network of cancer providers in North Central London, North East London and West Essex (initially known as ‘London Cancer’, population 3.2 million) worked to centralise specialist surgery services for eight cancer pathways across urology, head and neck, brain, OG and haematological cancer services [18]. Cardiovascular service delivery was also reconfigured around this time [19]. Delays in implementing the GM reconfigurations meant the health economics and other quantitative work covered only LC [16, 17, 20, 21].
This paper reports the results of an economic evaluation of the reorganisation of specialist prostate, bladder, renal and OG cancer surgery services in the LC region, compared to non-reconfigured services. We conducted a difference-in-differences analysis of observational patient-level electronic health records, to calculate mean adjusted and discounted per-patient costs and outcomes over a 10-year time horizon for patients receiving surgery. We report the net monetary benefit (NMB) of the LC reconfiguration and the Rest of England excluding Greater Manchester (ROE) for each cancer, and their incremental costs and incremental quality-adjusted life-years (QALYs).
2 Methods
2.1 Outline and Cohorts
This analysis used routine data from the National Cancer Registration and Analysis Service (NCRAS), which covers all cancer patients in England [22]. We included those diagnosed with one of the four cancers of interest between 2012 and 2017, who had had specialist surgery for the relevant cancer. We controlled for time trends using difference-in-differences methodology and adjusted for possible confounding. This cost-effectiveness analysis (CEA) mirrors the statistical analysis of clinical outcomes [17, 21].
We note that the main quantitative analysis [21] suggests that there were statistically significant reductions in LOS in prostate, bladder and renal cancers after the reconfigurations using the same structure of a difference-in-differences analysis, and increases in per-surgeon volumes of specialist surgeries, but that there was no evidence for changes in mortality or readmissions, possibly due to the existing risk of this being low, meaning that the sample sizes were too small to show a significant change in these less common outcomes.
The difference-in-differences analysis categorised patients according to when and where they received surgery. ‘Before’ covered the time before reconfiguration had begun; ‘during’ began when some patients were starting to be treated under the new system; and ‘after’ began when all patients were expected to be receiving care under the new system (see Table 1). The during periods were omitted from the difference-in-differences analysis. The OG changes had no during period, as all changes took place on one day, 1 January 2016. The geographical categories were LC and the ROE. The LC group included patients receiving surgery at one of the 11 trusts within the LC network region as was (Barts and the Royal London; Barking, Queen’s, King George; Homerton; Royal Free London; North Middlesex; Princess Alexandra; University College London Hospitals; Whittington; Chase Farm and Barnet; Whipps Cross; and Newham). During the centralisations some trusts merged, and there were eight at the time of analysis. The LC reconfigurations involved the following moves: OG, from three sites to two; prostate and bladder, from four sites to one; and renal, from nine sites to one. The ROE group included patients treated at other London trusts and elsewhere in England, excluding those treated in private hospitals and those treated in GM, given the planned centralisations there. A more detailed description of how the reorganisations were carried out and what staffing, equipment and other changes were involved alongside their associated costs can be found in our earlier publication on the cost of implementing the change [20] and are also discussed in other RESPECT-21 publications [17, 18, 23].
The CEA used decision analytic models for each of the four time/place scenarios (LC before, LC after, ROE before, ROE after) across the four cancers (prostate, bladder, renal, OG), calculating costs (National Health Service [NHS] provider perspective) and outcomes (QALYs), summarised as the NMB for the reconfigured services in the LC area compared to non-reconfigured services using difference-in-differences, and presented as the probability of the reconfiguration having been cost-effective at a given threshold. Each model calculated costs and QALYs over a short-term decision tree, followed by a Markov model, for 1000 hypothetical patients with the same initial disease status and demographics as those in the linked patient-level dataset. This time horizon reflects the likely lifetime of the changes [20], and captured relevant costs and outcomes.
The adjustment variables were age on index operation date (in 10-year bands), sex, ethnicity (White, other, not known), Index of Multiple Deprivation (IMD) quintile, cancer tumour stage at diagnosis (T1, T2, T3, T4, TX, missing), combined Gleason grade for prostate cancer (low: less than 7; moderate: 7; high: more than 7; missing), tumour grade for the other three cancers (G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated; GX: grade not appropriate or cannot be assessed; missing), Charlson Comorbidity Index (0, 1, 2, 3) and total number of cancers diagnosed (1, 2, 3+). All adjustment variables were included for all four cancers in the decision trees, and were assessed for inclusion in the survival models and retained if their removal worsened the fit. The analyses were performed using Stata v17 and Excel, in UCL’s Data Safe Haven walled garden environment, which is certified to an ISO27001 information security standard and conforms to NHS Digital's Information Governance Toolkit.
2.2 Dataset
Patients diagnosed with any of the four cancers between 01/01/2012 and 31/12/2017 were identified in the NCRAS dataset, accessed via Public Health England’s Office for Data Release (ODR), using International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) codes (see the Electronic Supplementary Material, Section 1). The Cancer Registry data contained information on patient, diagnosis and disease characteristics, and were linked by NCRAS at the patient level to hospital inpatient [24], outpatient and accident and emergency (A&E) data from the Hospital Episode Statistics (HES) dataset of all hospital admissions in the English NHS, which contains information about patients’ operations and other procedures. The dataset was further linked by NCRAS to Office for National Statistics (ONS) mortality data. This analysis used a subset of this dataset where people had specialist surgery for their cancer as defined using OPCS codes (see the Electronic Supplementary Material, Section 2).
2.3 Model Structure Outline
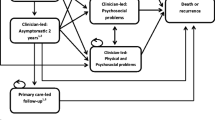
The design was similar to previous work on stroke centralisations [25], modelling patient pathways using a short-term decision tree followed by a longer-term state transition Markov model (see Fig. 1). The prostate decision model comprised a 90-day decision tree, and the renal, bladder and OG models comprised 30-day decision trees, all followed by 10-year Markov models with 6-monthly cycles. Differences in decision tree durations reflected differences in prognoses and commonly used outcome metrics. Patients entered the decision tree on the surgery date, and were classified at the end of the decision tree into one of three states: ‘healthy’, ‘not healthy’ or ‘dead’.
Patients were classified as ‘healthy’ in the prostate cancer model if their LOS for the index surgery was less than 3 days and they were not readmitted within 90 days with a primary diagnosis of prostate cancer. Prostate cancer patients who either had LOS longer than 3 days or were readmitted within 90 days, or both, were ‘not healthy’ at 90 days, and those who had died by 90 days after surgery, from any cause, were classified as ‘dead’. Those alive at the end of the decision tree moved into a two-state Markov model (see Fig. 1).
In the bladder, renal and OG cancers, patients were ‘healthy’ at the end of the 30-day decision tree if they were not readmitted to hospital with the primary diagnosis being the same cancer within 30 days, and were ‘not healthy’ if they were readmitted. Those who had died by 30 days after surgery, from any cause, were classified as ‘dead’ at the end of the decision tree. Patients alive at the end of the decision tree moved into a two-state Markov model (see Fig. 1).
In all cases, the routes of readmission were not considered; only the diagnosis code at readmission was used.
2.4 Costs
2.4.1 Treatment Event Costs (Hospital Episode Statistics Events)
Treatment event costs were calculated using inpatient, outpatient and A&E attendances from HES. Inpatient costs were categorised by Secondary Uses Service (SUS) Healthcare Resource Group (HRG) (corresponding to ‘Currency Code’ in NHS Reference Costs) and Class (ordinary admission, day case admission, regular day or night attender), with a standard unit cost per bed-day calculated according to these types by dividing the full consultant episode (FCE) cost by reported average LOS in the Reference Costs. This conversion of inpatient episode unit costs to an average unit cost per bed-day was performed in order to capture the cost impact of any LOS differences in the analysis. Outpatient unit costs were categorised by Treatment Specialty (corresponding to ‘Service Code’ in NHS Reference Costs), using published average costs that had been weighted according to national proportions of consultant-led and non-consultant-led attendances. A&E unit costs were categorised by A&E Department Type. Unit costs were applied as the latest available NHS Reference Costs from 2010/11 to 2017/18, adjusted to the 2018/19 financial year using the new Health Services (HS) Index, using Consumer Price Index (CPI) (Health) and the previous hospital and community health services (HCHS) indices for part-adjustment of older prices [26, 27]. The reference costs for 2018/19 were not used as their reporting format changed between 2017/18 and 2018/19 so that bed-day costs could no longer easily be calculated.
2.4.2 Cost of Implementation
The adjusted cost of designing, planning and implementing the LC reconfigurations was approximately £7.2 million in 2017–2018 prices, with significant investment in capital expenditure and staff time and effort, and a detailed description of our calculation is reported elsewhere [20]. To incorporate it into this analysis, the total implementation cost was annuitised assuming a lifetime of the assets and reconfigurations of 10 years and an interest rate of 3.5%, following Drummond et al. [28]. The annuitised rate was then divided by the total population and multiplied by the yearly incidence of the relevant disease [29], then costs were uplifted to 2018–2019 prices using the new HS Index, using CPI (Health) [27], leading to per-patient estimated implementation costs of £458, £375, £703 and £195 for prostate, bladder, renal and OG, respectively. These costs were added to costs for the ‘LC after’ group at the start of the decision tree, in the base-case analysis.
2.5 Quality-Adjusted Life-Years (QALYs) and Utilities
Patient-level health-related quality-of-life data were not available for this cohort as this information is not yet routinely collected in this context. Instead, assumptions were made regarding patients’ health states based on treatment events observed in the dataset, and appropriate utility scores from published studies were applied (see the Electronic Supplementary Material, Section 3) [30,31,32,33,34,35,36,37,38,39,40,41,42]. Searches were performed on 26 August 2020 for utility scores, focusing on National Institute for Health and Care Excellence (NICE) technology appraisal documentation [43] and the Tufts database (Center for the Evaluation of Value and Risk in Health) [44], and by snowballing to find other relevant work. Reported literature values were applied as follows: ‘healthy’ patients were approximated to ‘pre-progression’ patients in published studies, and ‘not healthy’ patients to ‘post-progression’ patients. Published utility values had been obtained in various ways, e.g. calculated from patient-completed questionnaires (EQ-5D [45], European Organization for Research and Treatment of Cancer [EORTC] [46], and SF-12 [47]), and estimated by experts where patient-reported information was unavailable. NICE recommends QALYs be calculated using utility scores generated by the EQ-5D [43], and the 3L was most common in the work we found, so we preferentially used this where available. We also preferentially included patients in standard-of-care arms in trials. Standard errors (SEs) reported in the literature varied, including some reports of zero (i.e. unknown) SEs in OG cancer, and some reports where the utility was given as a min–max range (0.5–1.0) in bladder cancer; so, a median SE of 0.1 was used for all utilities. The utility scores of the amalgamated ‘alive’ patients in the Markov models were the weighted means according to the overall relative proportions of healthy/not healthy patients at the end of each decision tree (see the Electronic Supplementary Material, Section 4). The utility value of the dead health state was zero. Utility scores in the decision tree were assigned for the whole 30- or 90-day period on the basis of the patient’s health state at the end of the decision tree.
2.6 Statistical Analysis
2.6.1 Decision Tree: Health State Proportions
Proportions of patients in the three health states at the end of the decision tree (healthy, not healthy, dead) were estimated separately for each of the four cancers using ordered logistic regression models, controlling for place (LC/ROE) and time period (before/during/after) using an interaction term, and adjusting for patient and disease characteristics listed in Sect. 2.1.
2.6.2 Decision Tree: Estimation of Costs
Decision tree costs per health state were calculated by summing the 30- or 90-day costs per patient and performing regression analysis, adjusting for the covariates described in Section 2.1. Mean (standard deviation) per-patient 30- or 90-day costs by health state, time period and region for inpatient, outpatient and A&E events were estimated using generalised linear models with a gamma distribution and log link.
2.6.3 Decision Tree: Calculation of QALYs
QALYs were calculated by multiplying the assigned utility score by the duration of the decision tree, for each health state, time period, region and cancer.
2.6.4 Markov Transition Probabilities: Survival Analysis
Parametric survival models using mortality data were fitted and the results used to calculate 6-month transition probabilities for the two-state Markov models for the four relevant difference-in-differences scenarios (LC before, LC after, ROE before, ROE after). The during groups were omitted. The censor date was the date of death or the latest follow-up time point for patients without a death date recorded. The start date was 30 or 90 (for prostate) days after the index surgery date. Exponential, Weibull and Gompertz distributions were assessed. Estimated numbers of deaths were compared with observed numbers of deaths at 6 months and 1 year after surgery, and, where estimated and observed values did not match, removal of time/place scenario variables as well as other adjustment covariates was assessed to improve model fit. The best fits were chosen based on visual comparison of the observed Kaplan-Meier survival curves and survival estimation curves (shape, ordering of curves by time/place scenario), as well as comparison of estimated and observed numbers of deaths.
2.6.5 Markov Cycles: Estimation of Costs and QALYs
Markov model 6-month cycle costs were calculated for alive patients using the unweighted mean across the four time/place scenarios of the outpatient decision tree costs, then dividing by the number of days in the decision tree (30 or 90) and multiplying by 183 days. Only outpatient costs were included in the alive Markov cycle costs as patients would not be expected to maintain the same level of resource use over the subsequent 10 years as they had received during the decision tree, according to recommendations in the relevant NICE guidelines for each cancer [39, 49,50,51,52]. Our cost estimates were similar to values in the published literature for similar patient groups. For example, Cox et al. provided estimates with a mean of £1385 (2017 prices) over 6 months when considering follow-up treatment over 3 years following surgery in the BOXIT trial [50].
The one-off costs for death in the urology cancer Markov models were taken from published values for prostate cancer, and in the OG Markov model, these were taken from published values for colorectal cancer [53], uplifted to 2018–2019 prices as before [27].
QALYs were calculated by multiplying the utility score by the cycle length in years and the number of patients in that health state, and then summing this over the 10-year horizon, for each health state, time period, region and cancer.
2.6.6 Probabilistic Sensitivity Analysis
The overall 10-year adjusted and discounted per-patient incremental costs and QALYs for the LC reconfigurations compared to non-reconfigured services were calculated by summing the costs and QALYs from the decision tree and Markov sections for each cancer. A 3.5%/year discount rate was used for both costs and QALYs [43]. Monte Carlo simulations generated estimates of costs and QALYs and 95% credible intervals (CIs), using the 2.5th and 97.5th percentiles of the calculated difference-in-differences costs and utilities [54]. Probabilistic distributions (gamma distributions for costs, beta distributions for utilities and log for survival) were used to account for parameter uncertainty, with 5000 iterations per cancer.
The results were plotted on cost-effectiveness planes (CEPs), and translated onto cost-effectiveness acceptability curves (CEACs), for a range of cost-effectiveness thresholds (£0–80,000 per QALY gained). The NMB was calculated for each of the four time/place scenarios of interest at commonly used thresholds: NICE threshold boundaries of £20,000 and £30,000 [43], and an efficiency threshold of £13,000 [55], as this lower threshold could be of interest to (local) decision makers in the context of this type of service reorganisation planning.
2.6.7 Deterministic Sensitivity Analysis
We conducted a range of sensitivity analyses, excluding implementation costs, and varying assumptions around reconfiguration timings, and input parameters and their estimation. We assessed inclusion of those from the ‘during’ period within the ‘after’ period, and tested different cost imputation decisions where models for predicting costs in the decision tree failed to converge due to small patient numbers.
3 Results
3.1 Population
Tables in the Electronic Supplementary Material, Section 5, show patient and disease characteristics at surgery date for each cancer, by region and time period. Sample sizes are repeated in Table 2. There is broad consistency across most categories, except for ethnicity and deprivation; there is a greater proportion of White patients in ROE than in LC, and worse deprivation in patients in LC than in ROE. We also note that according to the Gleason combined score in patients receiving specialist prostate cancer surgery, those in the ‘after’ groups had more severe disease than those in ‘before’ groups. This could be due to less severe patients being offered other non-surgical treatments and more severe patients being increasingly offered specialist surgery, after the reconfiguration. Sample sizes after reconfiguration were small, especially for bladder and OG, limiting the conclusions that can be drawn and leading to uncertainty in results.
3.2 Overall Cost-Effectiveness Results
The overall 10-year, adjusted, discounted cost-effectiveness results for the four cancers are reported in Table 2. There was a 79% probability that the LC reconfigurations were cost-effective for prostate cancer at cost-effectiveness threshold £30,000/QALY gained, 62% probability for OG cancer at the same threshold, 49% for bladder cancer and 12% for renal. Figure 2 shows the CEPs and CEACs, and the NMBs are reported in Table 2. The CEPs illustrate the uncertainty, with the position of the cloud of points indicating cost-effectiveness or otherwise. The proportion of points lying below the £30,000/QALY cost-effectiveness threshold (straight purple line in CEP with 30,000 gradient) corresponds to the probability of cost-effectiveness at that threshold, i.e. most (79%) of the points in the prostate CEP are below the threshold, and only a few (12%) of the points in renal CEP are below.
Cost-effectiveness planes (CEPs) and cost-effectiveness acceptability curves (CEACs) for prostate, bladder, renal and OG. LC reconfigurations compared to the ROE using difference-in-differences methodology, 10-year horizon, adjusted and discounted. (Purple straight lines on CEP: £30,000 cost-effectiveness threshold; red diamonds in centre of CEP: point showing mean difference-in-differences incremental costs and QALYs.) LC London Cancer, OG oesophago-gastric, QALY quality-adjusted life-year, ROE Rest of England excluding Greater Manchester
Table 2 also gives the incremental costs and incremental QALYs for each of LC and ROE separately (after vs. before) and for the overall difference-in-differences (‘LC after’ vs. non-reconfigured). The 95% CIs are given in the Electronic Supplementary Material, Section 6. These figures show that the cost per surgical patient became more expensive for all cancers in both regions, with significant increases for all except bladder, which also had the smallest sample size. Incremental QALYs also increased for all cancers in both regions, being statistically significant for prostate, and for renal and OG in ROE only. Positive NMB suggests good value for money (i.e. > 50% probability of cost-effectiveness) at a given threshold, and the prostate reconfigurations gave a positive NMB at all thresholds, and OG at the higher two thresholds. These results can be transformed into values per annual LC cohort, by multiplying by the annual incidence figures [29]. These cohort values are given in Table 3.
3.3 Sensitivity Analysis
The main secondary analysis involved removing the implementation costs, and the results for this are given in tables and plots in the Electronic Supplementary Material, Section 10. The likelihoods of the reconfigurations being cost-effective were all slightly higher for each cancer due to the slightly lower costs in the ‘LC after’ group, but the overall conclusions remained the same.
Including those from the ‘during’ period within the ‘after’ period had no impact on the conclusions described above. Use of various slightly different imputation assumptions for missing decision tree costs due to lack of convergence in some cases due to small patient numbers (for example, in A&E use) also had no impact on the conclusions.
4 Discussion
For prostate cancer, care delivered to patients undergoing specialist surgery via the reconfigured service was both more expensive and better (in QALY terms) than in the non-reconfigured service, and there was a 79% probability that this was cost-effective at a threshold of £30,000/QALY gained (base case including the implementation costs). The picture was similar in OG (62% at £30,000/QALY threshold). For the bladder reconfigurations (49% probability of having been cost-effective), the sample size was small, and the calculated differences in costs and QALYs were small, as can be seen in Fig. 2, where the central point in the CEP sits almost at the origin. For renal, however, the cloud of points in the CEP is in the northwest quadrant, suggesting that the reconfigured services (LC after) were dominated by the non-reconfigured system, in that they cost relatively more and gave relatively fewer QALYs. This appears to be driven by a large increase in QALYs in ROE, making the LC region appear worse off over this time period even though it did also lead to increased QALYs per patient. This highlights the importance of taking into account contemporaneous changes that happen elsewhere when conducting observational service evaluations.
Previous work in some specialist or acute services provided evidence that there can be benefits in these types of centralisation in terms of patient outcomes, for example, acute stroke [1, 6, 7] and some cancers [2, 3]. It is important to note, however, that this is not the same context as more mainstream services such as maternity care, where the risks and benefits of centralisation differ. It is also possible that any benefits from centralisations due to economies of scale might be more evident in more common conditions, for example, in more common cancers such as prostate, suggesting that centralising specialist services in less common cancers might be less likely to be cost-effective; however, we cannot know this for certain from this analysis due to the small patient numbers captured during this timeframe. For decision-makers who may be considering reconfiguring cancer services in their region, when using cost-effectiveness as a criterion, they therefore may consider specialist prostate services as a potential contender based on the results of this analysis, although with the caveat of significant uncertainty. Also, the context in which the reconfigurations occurred means that the results for prostate cancer may not be considered in isolation from the other three cancers; the results for prostate cancer may not have occurred in this way if the other changes had not taken place at the same time. This is because the delivery of healthcare services in the NHS is a highly networked and collaborative activity, as we have seen in particular during the coronavirus disease 2019 (COVID-19) pandemic and as discussed in related work from the RESPECT-21 study team [18]. When the reconfigurations were taken together over the 10-year horizon and over a cohort of 3413 surgery patients across the four cancers, based on the annual incident rate of each cancer, they resulted in an extra £2.3 million cost to the LC region providers, and resulted in 102 more QALYs for this group overall (see Table 3).
4.1 Strengths and Weaknesses
We used a comprehensive, nationwide dataset with detailed patient-level information on patient and disease characteristics, and hospital visits, treatments and mortality. We did not have explicit information on cancer progression status over the 10-year period, except indirectly in the ‘not healthy’ group during the decision tree, and the use of a single ‘alive’ health state in the Markov section of the model is a limitation of the analysis. Randomised controlled trials are the gold standard to control for potential confounders, but they are rarely possible in service change evaluations, so, we used difference-in-differences methodology, adjusting for available baseline patient and disease characteristics recommended by the clinical authors and other clinical colleagues, to control for other contemporaneous changes that might have taken place [56]. Sites in GM were excluded from the ROE group to accommodate the parallel trends assumption in difference-in-differences analysis, and this was acceptable because the main statistical analysis performed pre-trends tests to ascertain whether any difference in the linear trends in case-mix adjusted outcomes was apparent, when comparing values in the LC region with those in the ROE before the centralisations. Further details can be found in that work, but it was found, for all 27 outcomes assessed, that the coefficient of the interaction term of region and linear time trend was non-significant (p > 0.05) except for the binary variable indicating whether LOS was longer than 3 days in specialist prostate cancer surgery [21].
Patient-reported health-related quality-of-life data are not routinely collected in this context so estimates of utility scores were obtained from published sources and applied to patients in the model. It is possible that not all impacts on clinical outcomes were fully reflected in the QALY values, due to the lack of patient-level utility data in the dataset and to the small number of health states used in the decision tree and Markov portions of the models. We were also limited in only including patients who received specialist surgery, so, if changes in the delivery of specialist surgery led to differences in non-surgical treatments being offered, this was not captured in this analysis beyond controlling for patient and disease baseline characteristics.
Our analysis was restricted to an NHS payer perspective as we had resource use information from hospital records and not primary care or community services. The impact of this depends on the cancer and relative importance of wider costs. The impact of making a sub-optimal decision regarding any future decisions to reorganise other similar services could be quantified by performing a value of information (VOI) analysis to quantify the decision uncertainty relating to the absence of this information and other types of information. These other types of information could involve obtaining a larger sample size and the various costs associated with obtaining more data, as well as having other non-hospital data, alongside the benefits of having more data with which to populate the analysis and thus avoid a poor decision. We did not, however, perform a VOI analysis. We were also unable to include wider economic costs such as impact on employment in this analysis.
The timelines of the research programme meant that we only had data for patients diagnosed up to the end of 2017, so, ongoing learning and efficiencies could not be included in the analysis. The limited timeframe also resulted in small patient cohorts. Some models did not converge, so, conservative estimates for missing decision tree cost information were made, which could have underestimated some of the differences in costs. Small patient numbers also reduce the ability to detect significant differences and mean that outliers can have a greater impact on the results.
4.2 Comparison with Other Studies
This type of economic analysis, calculating the incremental cost per QALY gained, is not common in economic evaluations of service reconfiguration [8, 14, 15], meaning this is one of the few cost-utility analyses in this area. Greving et al. developed a decision model to evaluate the centralisation of ovarian cancer services in hospitals in the Netherlands; services provided in semi-specialised hospitals compared to general hospitals were cost-effective at €7135/QALY gained, but tertiary hospital care was not (€102,642/QALY) [57]. The majority of evaluations in this area focus on the relationship between hospital patient volume and clinical outcomes, with some evidence that concentrating procedures in high-volume hospitals is related to improved clinical outcomes [2, 3, 58]. The methodology of many of these studies, though, means that caution should be exercised when interpreting and comparing these results [8].
4.3 Conclusions and Implications
There is some evidence for cost-effectiveness of the LC reconfigurations, with the strongest being for prostate cancer. It is not clear, however, that the individual cancer reconfigurations could be implemented alone, especially as urology cancer pathways overlap clinically, so, perhaps the results of the four analyses need to be considered together. The results of this analysis should be interpreted with caution due to the small patient numbers and the number of assumptions made, and should be considered alongside the clinical evidence available. It would be important for future work to be able to more explicitly consider patient outcomes, for example, by routinely collecting brief patient-reported quality-of-life measures such as the EQ-5D-5L.
References
Fulop NJ, Ramsay AI, Perry C, Boaden RJ, McKevitt C, Rudd AG, Turner SJ, Tyrrell PJ, Wolfe CD, Morris S. Explaining outcomes in major system change: a qualitative study of implementing centralised acute stroke services in two large metropolitan regions in England. Implement Sci. 2016;11:80.
Coupland VH, Lagergren J, Lüchtenborg M, Jack RH, Allum W, Holmberg L, Hanna GB, Pearce M, Møller H. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut. 2013;966(62):961.
Nuttall M, van der Meulen J, Phillips N, Sharpin C, Gillatt D, McIntosh G, Emberton M. A systematic review and critique of the literature relating hospital or surgeon volume to health outcomes for 3 urological cancer procedures. J Urol. 2004;172:2145–52.
Fulop N, Walters R, Perri P, Spurgeon P. Implementing changes to hospital services: factors influencing the process and ‘results’ of reconfiguration. Health Policy. 2012;104:128–35.
Darzi A. Healthcare for London: a framework for action. 2nd ed. London: NHS London; 2007.
Morris S, Hunter RM, Ramsay AIG, Boaden R, McKevitt C, Perry C, Pursani N, Rudd AG, Schwamm LH, Turner SJ, Tyrrell PJ, Wolfe CDA, Fulop NJ. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ Open. 2014;349: g4757.
Ramsay AIG, Morris S, Hoffman A, Hunter RM, Boaden R, McKevitt C, Perry C, Pursani N, Rudd AG, Turner SJ, Tyrrell PJ, Wolfe CDA, Fulop NJ. Effects of centralizing acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke. 2015;46:2244–51.
Bhattarai N, McMeekin P, Price C, Vale L. Economic evaluations on centralisation of specialised healthcare services: a systematic review of methods. BMJ Open. 2016;6: e011214.
Posnett J. The hospital of the future: is bigger better? Concentration in the provision of secondary care. BMJ. 1999;319:1063–5.
Imison C, Sonola L, Honeyman M, Ross S. The reconfiguration of clinical services. What is the evidence? London: The King’s Fund; 2014.
Hoomans T, Severens JL. Economic evaluation of implementation strategies in health care. Implement Sci. 2014;9:168.
Severens JL, Hoomans T, Adang E, Wensing M. Economic evaluation of implementation strategies. In: Wensing M, Grol R, Grimshaw J, editors. Chapter 23 of improving patient care: the implementation of change in health care. 3rd ed. Oxford: Wiley; 2020.
Vale L, Thomas R, MacLennan G, Grimshaw J. Systematic review of economic evaluations and cost analyses of guideline implementation strategies. Eur J Health Econ. 2007;8:111–21.
Meacock R. Methods for the economic evaluation of changes to the organisation and delivery of health services: principal challenges and recommendations. Health Econ Policy Law. 2019;14:119–34.
Ke KM, Hollingworth W, Ness AR. The costs of centralisation: a systematic review of the economic impact of the centralisation of cancer services. Eur J Cancer Care. 2012;21:158–68.
Fulop NJ, Ramsay AIG, Vindrola-Padros C, Aitchison M, Boaden RJ, Brinton V, Clarke CS, Hines J, Hunter RM, Levermore C, Maddineni SB, Melnychuk M, Moore CM, Mughal MM, Perry C, Pritchard-Jones K, Shackley DC, Vickers J, Morris S. Reorganising specialist cancer surgery for the twenty-first century: a mixed methods evaluation (RESPECT-21). Implement Sci. 2016;11:155.
Fulop NF, Ramsay AIG, Vindrola-Padros C, Clarke CS, Hunter RM, Black GB, Wood VJ, Melnychuk M, Perry C, Vallejo-Torres L, Ng P-L, Barod R, Bex A, Boaden R, Bhuiya A, Brinton V, Fahy P, Hines J, Levermore C, Maddineni S, Mughal MM, Pritchard-Jones K, Sandell J, Shackley DC, Tran M and Morris S. Centralisation of specialist cancer surgery services in two areas of England: the RESPECT-21 mixed-methods evaluation. Health Soc Care Deliv Res. 2022 (In press).
Vindrola-Padros C, Ramsay AI, Perry C, Darley S, Wood VJ, Clarke CS, Hines J, Levermore C, Melnychuk M, Moore CM, Morris S, Mughal MM, Pritchard-Jones K, Shackley D, Fulop NJ. Implementing major system change in specialist cancer surgery: the role of provider networks. J Health Serv Res Policy. 2020;26:4–11.
England NHS. Improving specialist cancer and cardiovascular services in North and East London. West Essex: Business Case; 2014.
Clarke CS, Vindrola-Padros C, Levermore C, Ramsay AI, Black GB, Pritchard-Jones K, Hines J, Smith G, Bex A, Mughal M, Shackley D, Melnychuk M, Morris S, Fulop NJ, Hunter RM. How to cost the implementation of major system change for economic evaluations: case study using reconfigurations of specialist cancer surgery in part of London, England. Appl Health Econ Health Policy. 2021;19(6):797–810.
Melnychuk M, Clarke CS, Hunter RM, Ramsay AI, Vindrola-Padros C, Black GB, Levermore C, Sandell J, Barod R, Bex A, Hines J, Mughal MM, Pritchard-Jones K, Tran MG, Maddineni S, Shackley D, Ng P-L, Fulop NJ, Morris S. Impact of major system change in specialist cancer surgery in London, UK: difference-in-differences analysis. 2022 (Submitted).
Henson KE, Elliss-Brookes L, Coupland VH, Payne E, Vernon S, Rous B, Rashbass J. Data resource profile: national cancer registration dataset in England. Int J Epidemiol. 2020;49:16–16h.
Black GB, Wood VJ, Ramsay AI, Vindrola-Padros C, Perry C, Clarke CS, Levermore C, Pritchard-Jones K, Bex A, Tran MG, Shackley DC, Hines J, Mughal MM, Fulop NJ. Loss associated with subtractive health service change: the case of specialist cancer centralization in England. J Health Serv Res Policy. 2022. https://doi.org/10.1177/13558196221082585.
Herbert A, Wijlaars L, Zylbersztejn A, Cronwell D, Hardelid P. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol 2017;46(4):1093–1093i. https://doi.org/10.1093/ije/dyx015.
Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. Pharmacoeconomics. 2015;33(4):355–66.
Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury, 2015.
Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury, 2018.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: OUP; 2015.
Public Health England (PHE). Personal communication, CancerStats, PHE; Number of newly diagnosed patients in NCEL and West Essex in 2017; 2019.
Clarke CS, Hunter RM, Gabrio A, Brawley CD, Ingleby FC, Dearnaley DP, Matheson D, Attard G, Rush HL, Jones RJ, Cross W, Parker C, Russell JM, Millman R, Gillessen S, Malik Z, Lester JF, Wylie J, Clarke NW, Parmar MK, Sydes MR, James ND. Cost-utility analysis of adding abiraterone acetate plus prednisone/prednisolone to long-term hormone therapy in newly diagnosed advanced prostate cancer in England: Lifetime decision model based on STAMPEDE trial data. PLoS ONE. 2022;17(6): e0269192.
Sutton AJ, Lamont JV, Evans RM, Williamson K, O’Rourke D, Duggan B, Sagoo GS, Reid CN, Ruddock MW. An early analysis of the cost-effectiveness of a diagnostic classifier for risk stratification of haematuria patients (DCRSHP) compared to flexible cystoscopy in the diagnosis of bladder cancer. PLoS ONE. 2018;13(8): e0202796.
Kulkarni GS, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, Alibhai SM. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4(9): e284.
National Institute for Health and Care Excellence (NICE), Developing NICE guidelines: the manual (PMG20); 2014, updated 2018.
National Institute for Health and Care Excellence. Lenvatinib with everolimus for previously treated advanced renal cell carcinoma (NICE Guidance TA498); 2018. [Online]. www.nice.org.uk/guidance/ta498.
National Institute for Health and Care Excellence. Everolimus for advanced renal cell carcinoma after previous treatment (NICE Guidance TA432); 2017. [Online]. www.nice.org.uk/guidance/ta432.
National Institute for Health and Care Excellence. Nivolumab for previously treated advanced renal cell carcinoma (NICE Guidance TA417); 2016. [Online]. www.nice.org.uk/guidance/ta417.
Lee L, Sudarshan M, Li C, Latimer E, Fried GM, Mulder DS, Feldman LS, Ferri LE. Cost-effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Ann Surg Oncol. 2013;20:3732–9.
Graham AJ, Shrive FM, Ghali WA, Manns BJ, Grondin SC, Finley RJ, Clifton J. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg. 2007;83(4):1257–64.
National Institute for Health and Care Excellence. Oesophago-gastric cancer: assessment and management in adults (NICE Guideline NG83); 2018. [Online]. www.nice.org.uk/guidance/ng83.
National Institute for Health and Care Excellence. Regorafenib for previously treated unresectable or metastatic gastrointestinal stromal tumours (NICE Guideline TA488); 2017. [Online]. www.nice.org.uk/guidance/ta488.
National Institute for Health and Care Excellence. Trastuzumab for the treatment of HER2-positive metastatic gastric cancer (NICE Guideline TA208); 2010. [Online]. www.nice.org.uk/guidance/ta208.
National Institute for Health and Care Excellence. Sunitinib for the treatment of gastrointestinal stromal tumours (NICE Guideline TA179); 2009. [Online]. www.nice.org.uk/guidance/ta179.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal’ 2013. [Online]. http://publications.nice.org.uk/pmg9. Accessed Jan 2015.
Center for the Evaluation of Value and Risk in Health. The Cost-Effectiveness Analysis Registry,” Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, USA, [Online]. www.cearegistry.org. Accessed 2019.
Dolan P. Modelling valuations for EuroQol health states. Med Care. 1997;35:1095–108.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechter H, Fleishman SB, de Haes JC, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Ware J, Kosinski M, Keller S. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
Clarke CS. Personal communication based on STAMPEDE patient-level data in control arm (recruited 2011–2014); 2020.
National Institute for Health and Care Excellence (NICE). Bladder cancer: diagnosis and management: NICE guideline NG2; 2015. [Online]. www.nice.org.uk/guidance/ng83. Accessed Dec 2020.
Cox E, Saramago P, Kelly J, Porta N, Hall E, Tan WS, Sculpher M, Soares M. Effects of bladder cancer on UK healthcare costs and patient health-related quality of life: evidence from the BOXIT trial. Clin Genitourin Cancer. 2019;18(4):e418-442.
Camp C, O’Hara J, Hughes D, Adshead J. Short-term outcomes and costs following partial nephrectomy in England: a population-based study. Eur Urol Focus. 2018;4:579–85.
Russell IT, Edwards RT, Gliddon AE, Ingledew DK, Russell D, Whitaker R, Yeo ST, Attwood SE, Barr H, Nanthakumaran S, Park KG. Cancer of Oesophagus or Gastricus—new Assessment of Technology of Endosonography (COGNATE): report of pragmatic randomised trial. Health Technol Assess. 2013. https://doi.org/10.3310/hta17390.
Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med. 2015;29(10):899–907.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, Devlin N, Smith PC, Sculpher M. Methods for the estimation of the NICE cost effectiveness threshold. Health Technol Assess. 2015;19(14):1–503.
Franklin M, Lomas J, Richardson G. Conducting value for money analyses for non-randomised interventional studies including service evaluations: an educational review with recommendations. Pharmacoeconomics. 2020;38:665–81.
Greving JP, Vernooij F, Heintz AP, van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effectiveness analysis. Gynecol Oncol. 2009;113(1):68–74.
Lopez Ramos C, Brandel MG, Steinberg JA, Wali AR, Rennert RC, Santiago-Dieppa DR, Sarkar RR, Pannell JS, Murphy JD, Khalessi AA. The impact of traveling distance and hospital volume on post-surgical outcomes for patients with glioblastoma. J Neuro-Oncol. 2019;141:159–66.
Acknowledgements
The authors acknowledge the contribution made to this work by Neil Cameron, one of the patient representatives on this study, who sadly died in May 2017. We also thank Michael Aitchison, Georgia Black, Veronica Brinton, Mark Emberton, Patrick Fahy, David Holden, Colin Jackson, Caroline Moore, Michelle Morton, Dipankar Mukherjee, Pei Li Ng, Catherine Perry, John Sandell, Christine Taylor and Ed Wilson for their contributions to the development of this study. The models and analysis code used in this study were provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The authors wish to thank the thoughtful and constructive journal editors and peer reviewers who provided their valuable insights to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This paper presents independent research commissioned by the National Institute for Health and Care Research (NIHR) Health Services and Delivery Research Programme, funded by the Department of Health and Social Care (study reference 14/46/19). NJF is an NIHR Senior Investigator. CVP and NJF are in part supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. KPJ was funded by UCLPartners Academic Health Science Network as Cancer Programme Director and as Chief Medical Officer of London Cancer from 2011 to 2016 when the latter funding reverted to the National Cancer Vanguard programme held locally by the University College London Hospitals (UCLH) Cancer Collaborative on behalf of all acute provider trusts in North Central and North East London and West Essex. London Cancer received funding from National Health Service (NHS) England (London Region). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Conflict of interest
Besides the funding received by authors’ institutions from the NIHR for this work as mentioned in the ‘Funding’ section, KPJ was Chief Medical Officer for London Cancer during the period of the changes described in this paper. MT has received payment or honoraria for teaching and educational events from Boston Scientific Corporation, grants from the NIHR paid to her institution, and payment for expert testimony as an external clinical advisor from the Parliamentary ombudsman for health services, and is a Trustee of Kidney Cancer UK and Governor of Royal Free London NHS Foundation Trust. DCS has received grants or contracts from CRUK for the ACED project, regarding research into early detection of cancer (A27859), and held a director role in the Greater Manchester Cancer Alliance (a NHS England cancer alliance). NJF has received grants or contracts from The Health Foundation and THIS Institute paid to her institution, and holds the role of Non-Executive Director, Whittington Health NHS Trust, and is a Trustee of Health Services Research UK. AIGR is also a Trustee of Health Services Research UK. All other authors have nothing to disclose.
Availability of data and material
The datasets generated and analysed during the current study are not shareable due to confidentiality.
Ethics approval
This study was reviewed and approved by the Proportionate Review Subcommittee of the NRES Committee Yorkshire and the Humber—Leeds (reference number 15/YH/0359).
Author contributions
CSC led the analysis, design and interpretation and wrote the first draft of the paper; MMe, AIGR, CVP, DCS, SM, NJF and RMH conceived of the initial study design; MMe, SM and RMH assisted in the analysis; AIGR, CVP, SM, NJF and RMH interpreted the results; RB, AB, JH, MMu, KPJ and MT provided clinical and health services input; CL provided health services input; NJF led the overall RESPECT-21 mixed methods programme of research. All authors contributed to the drafting of the paper and approved the final version.
Consent to participate
Not applicable as this analysis used anonymised routinely collected data.
Consent for publication (from patients/participants)
Not applicable.
Code availability
Analysis code can be made available on request to the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Clarke, C.S., Melnychuk, M., Ramsay, A.I.G. et al. Cost-Utility Analysis of Major System Change in Specialist Cancer Surgery in London, England, Using Linked Patient-Level Electronic Health Records and Difference-in-Differences Analysis. Appl Health Econ Health Policy 20, 905–917 (2022). https://doi.org/10.1007/s40258-022-00745-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00745-w