Abstract
Background
Itch is the most bothersome symptom reported by patients with psoriasis. Safe and effective treatments for psoriasis that also address itch are needed.
Objectives
To report effects of roflumilast cream on itch-related outcomes from a Phase 2b trial.
Methods
Adults with chronic plaque psoriasis were randomized to roflumilast 0.3%, roflumilast 0.15%, or vehicle once-daily for 12 weeks. Psoriasis severity was assessed via the Investigator Global Assessment (IGA; a 5-point scale assessing plaque thickening, scaling, and erythema ranging from 0 [clear] to 4 [severe]) and ≥ 2 on a modified Psoriasis Area and Severity Index (PASI-HD, which combines severity of lesions and area affected, ranging from 0 [no disease] to 72 [maximal disease], with the actual percentage of the anatomical area involved in those patients with < 10% of anatomical area involved [e.g., 0.1 for 1% to 0.9 for 9%]). Itch was evaluated via Worst Itch Numeric Rating Scale (WI-NRS), Psoriasis Symptom Diary (PSD) Items 1 (severity of itch) and 2 (bother of itch), and itch-related sleep loss NRS scores. Post hoc correlation analyses between WI-NRS and PASI, WI-NRS and itch-related sleep loss, and WI-NRS and DLQI were also performed.
Results
Roflumilast-treated patients had significantly greater improvements than vehicle-treated patients in WI-NRS and PSD Items 1 and 2 beginning at Week 2 and in itch-related sleep loss Weeks 6 through 12. Among patients with baseline WI-NRS ≥ 6, significantly more patients achieved ≥ 4-point improvement with roflumilast than with vehicle as early as Week 2. Itch severity had low correlation with PASI while WI-NRS and IGA were not always aligned.
Limitations
The first assessment was at 2 weeks, limiting the ability to assess early onset of itch response.
Conclusion
Roflumilast cream improved itch and itch-related sleep loss associated with chronic plaque psoriasis.
ClinicalTrials.gov identifier
NCT03638258.
Similar content being viewed by others
Itch is the most bothersome symptom of psoriasis and contributes to disease severity. |
Roflumilast cream may be an effective treatment for psoriasis, improving itch and itch-related sleep loss. |
1 Introduction
Itch is an especially common and important symptom of psoriasis [1,2,3]. Between 60 to 90% of patients with psoriasis report pruritus, and many identify itch as their most troublesome symptom [1, 2, 4,5,6,7,8,9]. Although usually limited to lesions, about 25% of patients experience generalized itching in uninvolved skin [8]. Pruritus severity is not always correlated with severity of psoriasis [8, 9].
Itching can impair quality of life, increase feelings of agitation, embarrassment, and stigmatization, and contribute to stress-related disorders, anxiety, and depression in patients with psoriasis [1, 10]. Patients report itching affects concentration, physical activity, and work/school attendance, and 50% report itching all or most of the time [2, 4]. Itch from psoriasis can disrupt sleep, resulting in less sleep than usual and difficulty in waking and feeling well-rested [1, 2]. Additionally, scratching may worsen psoriasis via koebnerization.
The mainstays of psoriasis treatment have varying efficacy in treating itch [1]. Current topical treatments for psoriasis have limitations, such as stinging and burning on sensitive locations with vitamin A or D derivatives and various local side effects with long-term topical corticosteroids [11]. Safe and effective agents that treat psoriasis and the associated itch, particularly nonsteroidal treatment options, may provide a more complete management strategy.
Phosphodiesterase-4 (PDE-4) is a proinflammatory enzyme up-regulated in psoriatic skin compared with normal skin [12]. Phosphodiesterase-4 inhibition reduces several cytokines important in the pathogenesis of psoriasis, such as tumor necrosis factor, interferon-γ, interleukin-17, and interleukin-23 [13, 14]. Inhibition of PDE-4 reduces itching in mouse models of dermatoses through mechanistic pathways independent of the anti-inflammatory action of PDE-4 [15,16,17,18,19,20,21]. The oral PDE-4 inhibitor apremilast (approved for treatment of plaque psoriasis) and the topical PDE-4 inhibitor crisaborole (approved for treatment of mild to moderate atopic dermatitis) significantly improved itch in clinical trials for psoriasis and atopic dermatitis, respectively [18, 20, 22, 23]. Roflumilast is a selective, highly potent PDE-4 inhibitor, with greater affinity for PDE-4 and approximately 25- to > 300-fold more potency than the other marketed oral or topical PDE-4 inhibitors, apremilast and crisaborole, for inhibiting in vitro cytokine secretion from human leukocytes [13].
In a Phase 2b study evaluating efficacy and safety of once-daily roflumilast cream (0.3% and 0.15%) for plaque psoriasis for 12 weeks, roflumilast resulted in significantly higher percentages of patients with a score on the investigator global assessment (IGA) indicating Clear or Almost Clear status at 6 weeks versus vehicle (roflumilast 0.3%: 28%; roflumilast 0.15%: 23%; vehicle: 8%; p ≤ 0.004) [24]. Safety of roflumilast was similar to vehicle with low incidence of treatment-emergent adverse events, including application site pain [24]. Here, we report results of patient-reported outcomes (PROs) related to itch from that Phase 2b study.
2 Methods
2.1 Study Design
Full details about the methods, design, inclusion and exclusion criteria, and the primary efficacy and safety outcomes for this parallel-group, double-blind, vehicle-controlled Phase 2b clinical trial have been published previously [24]. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Council for Harmonisation and is registered on ClinicalTrials.gov (NCT03638258). Before enrollment of patients, the study protocol and informed consent form were reviewed and approved by an appropriate Institutional Review Board or Independent Ethics Committee. All patients provided written informed consent before screening.
Patients were randomized to roflumilast 0.3% cream, roflumilast 0.15% cream, or vehicle in a 1:1:1 ratio via a computer-generated list. Roflumilast cream or vehicle was applied once-daily to all psoriasis lesions for 12 weeks. Palms and soles of the feet were treated but not included in any measurements of efficacy.
2.2 Patients
Eligible patients were males and females aged ≥ 18 years who had plaque psoriasis of at least mild severity affecting 2–20% body surface area (excluding the scalp, palms, and soles). At screening, patients had to have a score ≥ 2 on the IGA (a 5-point scale assessing plaque thickening, scaling, and erythema ranging from 0 [clear] to 4 [severe]) and ≥ 2 on a modified Psoriasis Area and Severity Index (PASI-HD, which combines severity of lesions and area affected, ranging from 0 [no disease] to 72 [maximal disease], with the actual percentage of the anatomical area involved in those patients with < 10% of anatomical area involved [e.g., 0.1 for 1% to 0.9 for 9%]) [25].
Key exclusion criteria were excessive exposure of treated areas to natural or artificial sunlight, tanning bed, or other light emitting device; diagnosis of guttate, erythrodermic/exfoliative, palmoplantar involvement only, or pustular psoriasis; and use of oral roflumilast or other PDE-4 inhibitors within the previous 4 weeks.
2.3 Study Assessments
The PASI-HD assessment was performed to assess severity of psoriasis at screening; baseline; and Weeks 2, 4, 6, 8, and 12. Itch was assessed using the secondary efficacy endpoints of Worst Itch Numeric Rating Scale (WI-NRS) [26], Items 1 and 2 of the Psoriasis Symptom Diary (PSD), and Itch-Related Sleep Loss Numeric Rating Scale (NRS). The WI-NRS was determined by asking patients to assess their worst itch over the past 24 h on a scale of 0 (no itch) to 10 (worst imaginable itch) [27]. In those patients with a WI-NRS ≥ 6 at baseline, the percentage of those achieving a 4-point reduction in WI-NRS was also determined. The PSD is a 16-item (24-h recall) assessment that measures burden and severity of psoriasis signs and symptoms and impact on functional health [28, 29]. Item 1 of the PSD was “Overall, how severe was your psoriasis-related itching over the past 24 h?” and patients reported on a scale of 0 (no itching) to 10 (itching as bad as you can imagine) [28, 29]. Patients responded to Item 2 of the PSD by answering “Overall, how bothered were you by your psoriasis-related itching over the past 24 h?” on a scale of 0 (not bothered at all) to 10 (bothered as bad as you can imagine) [28, 29]. Itch-related sleep loss was evaluated on a scale from 0 (no itch-related sleep loss) to 10 (itch-related sleep loss as bad as it could be) over the previous 24 h. The Dermatology Life Quality Index (DLQI) is a simple 10-item questionnaire used to assess the psychosocial impact of dermatology diseases [30]. Itch-related assessments were performed at baseline and Weeks 2, 4, 6, 8, and 12. The DLQI was added as an amendment to the protocol; therefore, DLQI was evaluated only in a subset of patients.
2.4 Statistical Analysis
All statistical tests were two-sided at the 0.05 level of significance with no adjustment for multiple comparison. Efficacy analyses were performed on the intention-to-treat population. Achievement of a 4-point reduction in WI-NRS in patients with baseline WI-NRS ≥ 6 was analyzed with logistic regression with a factor of treatment group and the respective baseline score as a covariate. The other continuous endpoints were analyzed with an analysis of covariance with a factor of treatment group and respective baseline score as a covariate. The primary method of handling missing efficacy data was a mixture of linear interpolation and last observation carried forward (if the missing assessment was not followed by at least one observed assessment).
A post hoc analysis was performed to determine correlation between WI-NRS and PASI, WI-NRS and itch-related sleep loss, and WI-NRS and DLQI. Pearson correlation coefficients were calculated for these measures at baseline.
3 Results
3.1 Patients
A total of 331 patients were randomized to and received roflumilast 0.3% cream (n = 109 patients), roflumilast 0.15% cream (n = 113 patients), or vehicle (n = 109 patients). Baseline demographic characteristics were similar among groups (Table 1). Patient ages ranged from 18 to 89 years (mean: 53.9 years).
3.2 Study Assessments
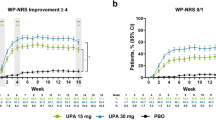
The overall baseline mean PASI score was 7.8 (Table 1). The percentage reduction from baseline for PASI was greater in roflumilast-treated groups than in the vehicle-treated group from Week 2 to Week 12 (all p < 0.001). Overall baseline mean scores for WI-NRS were similar across groups at 5.9 (Table 1). Patients in both roflumilast-treated groups had similar improvements in WI-NRS score. The least squares mean decrease (improvement) from baseline was greater for patients treated with roflumilast than for those treated with vehicle beginning at Week 2 (p ≤ 0.002; Fig. 1). Among the subgroup of patients with baseline WI-NRS ≥ 6, a significantly greater percentage of patients treated with roflumilast 0.3% achieved an improvement ≥ 4 points than among those treated with vehicle by the first timepoint measured, Week 2 (p ≤ 0.034; Fig. 2). For patients treated with roflumilast 0.15%, the percentage who achieved this level of improvement was greater than for those treated with vehicle at Week 6 (p = 0.012) and Week 12 (p < 0.001).
Least squares mean change in WI-NRS score over time (intention-to-treat population). Assessed on a scale from 0 (no itch) to 10 (worst imaginable itch). Missing data imputed using linear interpolation and last observation carried forward where linear interpolation was not computationally possible. CI confidence interval, LS least squares, WI-NRS Worst Itch Numeric Rating Scale. *Nominal p < 0.05 vs vehicle
Proportion of patients with WI-NRS score ≥ 6 at baseline who achieved a ≥ 4-point reduction from baseline. Assessed as the worst itch over the past 24 h on a scale ranging from 0 (no itch) to 10 (worst imaginable itch). Data are presented for intention-to-treat population. Missing data imputed using linear interpolation and last observation carried forward where linear interpolation was not computationally possible. CI confidence interval, WI-NRS Worst Itch Numeric Rating Scale. *Nominal p < 0.05 vs vehicle
For PSD, mean baseline scores for Item 1 (severity of itch) were similar across treatment groups (roflumilast 0.3%: 5.5; roflumilast 0.15%: 5.3; vehicle: 5.5; Table 1). Mean improvement was greater for patients treated with roflumilast than for those treated with vehicle at Weeks 2 to 12 (p ≤ 0.012; Fig. 3). Mean baseline scores for PSD Item 2 (bother of itch) were also similar across treatment groups (roflumilast 0.3%: 5.2; roflumilast 0.15%: 5.2; vehicle: 5.5; Table 1) and mean decrease from baseline was greater for patients treated with roflumilast than for those treated with vehicle Weeks 2 to 12 (p ≤ 0.010; Fig. 3).
Least squares mean change in severity of itch (left panel) and bother of itch (right panel) over time (intention-to-treat population). Assessed on scales from 0 (no itch/not bothered at all) to 10 (itching/bothered as bad as you can imagine). Missing data imputed using linear interpolation and last observation carried forward where linear interpolation was not computationally possible. CI confidence interval, LS least squares, PSD Psoriasis Symptom Diary. *Nominal p < 0.05 vs vehicle
The overall baseline mean score for itch-related sleep loss was 3.1 and was similar across groups (Table 1). Both active treatment groups had similar improvements in itch-related sleep loss, and this was greater for patients treated with roflumilast at either dose than for those treated with vehicle beginning at Week 6 (p ≤ 0.022; Fig. 4). Overall baseline mean DLQI score was 8.0 (Table 1). The response for patient-reported quality of life (DLQI) was consistent with those observed on itch-related sleep loss with improvement observed at Week 6 for patients treated with roflumilast 0.3% (p = 0.045). At Week 12, the improvement in DLQI score (3.0 in patients treated with roflumilast 0.3% and 3.9 in patients treated with roflumilast 0.15%) was greater than in patients in the vehicle-treated group (1.2; p ≤ 0.036).
Least squares mean change in itch-related sleep loss over time (intention-to-treat population). Assessed on a scale from 0 (no itch-related sleep loss) to 10 (itch-related sleep loss as bad as it could be). Missing data imputed using linear interpolation and last observation carried forward where linear interpolation was not computationally possible. CI confidence interval, IRSL itch-related sleep loss, LS least squares. *Nominal p < 0.05 vs vehicle
3.3 Post Hoc Correlation Analyses
At baseline, WI-NRS and PASI were positively correlated in all treatment groups, although these correlations were low. Pearson’s correlation coefficients were 0.189 for roflumilast 0.3%, 0.282 for roflumilast 0.15%, and 0.205 for vehicle (p ≤ 0.05 for all correlations). At Week 12, Pearson’s correlation coefficients were 0.399 for roflumilast 0.3%, 0.334 for roflumilast 0.15%, and 0.558 for vehicle (p ≤ 0.05 for all correlations; Fig. 5). Similarly, patient-reported itch (WI-NRS) and physician-assessed disease severity (IGA) were not always aligned. At baseline, patients with low itch scores included those with moderate or severe disease, as measured by IGA, whereas patients with mild disease (IGA = 1 to 2) often reported considerable itch (WI-NRS ≥ 5). Worst Itch Numeric Rating Scale and itch-related sleep loss were positively correlated in all treatment groups. Pearson’s correlations were 0.548 for roflumilast 0.3%, 0.646 for roflumilast 0.15%, and 0.652 for vehicle (p < 0.001 for all). This pattern was also observed for correlations between WI-NRS and DLQI with positive correlations seen at baseline and Pearson’s correlations of 0.445, 0.617, and 0.422 for roflumilast 0.3%, roflumilast 0.15%, and vehicle, respectively (p < 0.001 for all).
4 Discussion
Evaluating PROs in clinical trials provides information about the impact psoriasis symptoms have on patients’ lives and can support regulatory approval and labeling [2]. Itch associated with psoriasis is one of the most important and bothersome symptoms as reported by patients with psoriasis [2]. Several PROs related to itch were assessed in this study. Patients treated with roflumilast had greater improvements in WI-NRS as well as severity and bother of itch (as measured by PSD) compared with those treated with vehicle. These improvements occurred by the first timepoint measured, Week 2, with further improvements occurring over the course of the study.
Recognizing that a statistical improvement in the WI-NRS scale may not necessarily mean the patient experienced a clinically meaningful improvement, we also evaluated the proportion of patients with at least a 4-point change in WI-NRS, which is considered a clinically and qualitatively meaningful improvement in itch among patients with moderate to severe plaque psoriasis [26]. In this study, among patients with baseline WI-NRS ≥ 6, more roflumilast-treated patients had an improvement ≥ 4 points than among vehicle-treated patients with a greater percentage of patients treated with roflumilast 0.3% found at the first timepoint evaluated, Week 2.
Itching or scratching associated with psoriasis severely impacts a patient’s sleep by affecting their ability to fall asleep and/or stay asleep [2, 31]. Patients with psoriasis report more impairments in sleep quality, latency, duration, and efficiency, experience more sleep disturbances, and use sleeping medications more than individuals without psoriasis [31, 32]. Impairment of sleep quality and duration, including difficulty sleeping due to itching or scratching, is associated with negative effects on quality of life among patients with psoriasis [2, 31]. A mediation modeling analysis of several studies shows that itch is the driving force in DLQI improvement in psoriasis [33]. In the current study, roflumilast cream reduced (improved) itch-related sleep loss and improved patient-reported quality of life (DLQI) compared with vehicle.
In the post hoc correlation analysis, WI-NRS was positively correlated with PASI, itch-related sleep loss, and DLQI at baseline; however, the correlation with PASI was low. Additionally, at baseline, IGA and WI-NRS were not always aligned, with some patients with mild disease experiencing considerable itch. These results are similar to other studies in which itch intensity was not or only weakly correlated with psoriasis severity but was correlated with sleep and quality of life [1, 7,8,9, 34]. Thus, patients may have considerable itch despite mild psoriasis and this suggests itch related to psoriasis is caused by multiple factors, including mediators from the nervous, endocrine, and immune systems that induce or aggravate itch in response to external stimuli and psychological stress in addition to inflammation of psoriatic lesions [35].
One limitation of this analysis was that the earliest timepoint at which efficacy was evaluated was Week 2 and any changes in itch prior to this timepoint were not captured. In addition, no adjustments for multiple comparisons or multiplicity were made for these secondary endpoints. These outcomes are reported as point estimates and unadjusted 95% confidence intervals without multiplicity adjustment. Next, DLQI was added as a protocol amendment, which resulted in DLQI being evaluated in only a subset of patients rather than the entire study population. Fourth, itch-related sleep loss was evaluated on a numeric rating scale only and not more extensive measures of insomnia or sleep quality such as the Athens Insomnia Scale or Pittsburgh Sleep Quality Index.
In conclusion, in this Phase 2b study, once-daily roflumilast cream significantly improved itch and itch-related sleep loss in patients with chronic plaque psoriasis. This suggests this potent PDE-4 inhibitor may be an effective topical treatment of these important symptoms associated with chronic plaque psoriasis.
References
Elewski B, Alexis AF, Lebwohl M, Stein Gold L, Pariser D, Del Rosso J, et al. Itch: an under-recognized problem in psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1465–76. https://doi.org/10.1111/jdv.15450.
Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62. https://doi.org/10.1186/1477-7525-7-62.
World Health Organization. Global report on psoriasis. Geneva: World Health Organization; 2016.
Dickison P, Swain G, Peek JJ, Smith SD. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206–9. https://doi.org/10.1111/ajd.12747.
Gupta MA, Gupta AK, Kirkby S, Weiner HK, Mace TM, Schork NJ, et al. Pruritus in psoriasis. A prospective study of some psychiatric and dermatologic correlates. Arch Dermatol. 1988;124:1052–7. https://doi.org/10.1001/archderm.124.7.1052.
Prignano F, Ricceri F, Pescitelli L, Lotti T. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9–13. https://doi.org/10.2147/ccid.s4465.
Reich A, Hrehorów E, Szepietowski JC. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm Venereol. 2010;90:257–63. https://doi.org/10.2340/00015555-0851.
Reich A, Szepietowski JC. Clinical aspects of itch: psoriasis. In: Carstens E, Akiyama T, editors. Itch: mechanisms and treatment. Frontiers in Neuroscience. Boca Raton: CRC Press/Taylor & Francis; 2014.
Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–73. https://doi.org/10.1046/j.1365-2133.2000.03829.x.
Yan D, Blauvelt A, Dey AK, Golpanian RS, Hwang ST, Mehta NN, et al. New frontiers in psoriatic disease research, part II: comorbidities and targeted therapies. J Investig Dermatol. 2021;141:2328–37. https://doi.org/10.1016/j.jid.2021.02.743.
Elmets CA, Korman NJ, Prater EF, Wong EB, Rupani RN, Kivelevitch D, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–70. https://doi.org/10.1016/j.jaad.2020.07.087.
Schafer PH, Truzzi F, Parton A, Wu L, Kosek J, Zhang LH, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753–63. https://doi.org/10.1016/j.cellsig.2016.01.007.
Dong C, Virtucio C, Zemska O, Baltazar G, Zhou Y, Baia D, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413–22. https://doi.org/10.1124/jpet.116.232819.
Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. https://doi.org/10.3389/fphar.2018.01048.
Andoh T, Kuraishi Y. Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J Dermatol Sci. 2014;76:206–13. https://doi.org/10.1016/j.jdermsci.2014.10.005.
Andoh T, Yoshida T, Kuraishi Y. Topical E6005, a novel phosphodiesterase 4 inhibitor, attenuates spontaneous itch-related responses in mice with chronic atopy-like dermatitis. Exp Dermatol. 2014;23:359–61. https://doi.org/10.1111/exd.12377.
Fowler E, Yosipovitch G. A new generation of treatments for itch. Acta Derm Venereol. 2020;100:36–44. https://doi.org/10.2340/00015555-3347.
Yosipovitch G, Reich A, Steinhoff M, Beselin A, Kent T, Dossenbach M, et al. Impact of ixekizumab treatment on itch and Psoriasis Area and Severity Index in patients with moderate-to-severe plaque psoriasis: an integrated analysis of two Phase III randomized studies. Dermatol Ther (Heidelb). 2018;8:621–37. https://doi.org/10.1007/s13555-018-0267-9.
Ishii N, Shirato M, Wakita H, Miyazaki K, Takase Y, Asano O, et al. Antipruritic effect of the topical phosphodiesterase 4 inhibitor E6005 ameliorates skin lesions in a mouse atopic dermatitis model. J Pharmacol Exp Ther. 2013;346:105–12. https://doi.org/10.1124/jpet.113.205542.
Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a Phase IIb randomized, controlled study. Health Qual Life Outcomes. 2013;11:82. https://doi.org/10.1186/1477-7525-11-82.
Wakita H, Ohkuro M, Ishii N, Hishinuma I, Shirato M. A putative antipruritic mechanism of the phosphodiesterase-4 inhibitor E6005 by attenuating capsaicin-induced depolarization of C-fibre nerves. Exp Dermatol. 2015;24:215–6. https://doi.org/10.1111/exd.12606.
Amgen Inc. OTEZLA (apremilast) prescribing information. Thousand Oaks: Amgen Inc.; 2020.
Pfizer Labs. EUCRISA (crisaborole) prescribing information. New York: Pfizer Labs; 2020.
Lebwohl MG, Papp KA, Stein Gold L, Gooderham MJ, Kircik LH, Draelos ZD, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229–39. https://doi.org/10.1056/NEJMoa2000073.
Papp KA, Lebwohl MG, Kircik LH, Pariser DM, Strober B, Krueger GG, et al. The proposed PASI-HD provides more precise assessment of plaque psoriasis severity in anatomical regions with a low area score. Dermatol Ther (Heidelb). 2021;11:1079–83. https://doi.org/10.1007/s13555-021-00572-2.
Kimball AB, Naegeli AN, Edson-Heredia E, Lin CY, Gaich C, Nikaï E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157–62. https://doi.org/10.1111/bjd.14464.
Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol. 2015;54:715–22. https://doi.org/10.1111/ijd.12645.
Lebwohl M, Swensen AR, Nyirady J, Kim E, Gwaltney CJ, Strober BE. The Psoriasis Symptom Diary: development and content validity of a novel patient-reported outcome instrument. Int J Dermatol. 2014;53:714–22. https://doi.org/10.1111/j.1365-4632.2012.05798.x.
Strober BE, Nyirady J, Mallya UG, Guettner A, Papavassilis C, Gottlieb AB, et al. Item-level psychometric properties for a new patient-reported psoriasis symptom diary. Value Health. 2013;16:1014–22. https://doi.org/10.1016/j.jval.2013.07.002.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
Kaaz K, Szepietowski JC, Matusiak Ł. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol. 2019;99:175–80. https://doi.org/10.2340/00015555-3065.
Melikoglu M. Sleep quality and its association with disease severity in psoriasis. Eurasian J Med. 2017;49:124–7. https://doi.org/10.5152/eurasianjmed.2017.17132.
Taylor PC, Bushmakin AG, Cappelleri JC, Young P, Germino R, Merola JF, et al. Itch as major mediator of effect of tofacitinib on health-related quality of life in psoriatic arthritis: a mediation analysis. J Clin Med. 2021;10:4081. https://doi.org/10.3390/jcm10184081.
Roblin D, Wickramasinghe R, Yosipovitch G. Pruritus severity in patients with psoriasis is not correlated with psoriasis disease severity. J Am Acad Dermatol. 2014;70:390–1. https://doi.org/10.1016/j.jaad.2013.09.030.
Komiya E, Tominaga M, Kamata Y, Suga Y, Takamori K. Molecular and cellular mechanisms of itch in psoriasis. Int J Mol Sci. 2020;21:8406. https://doi.org/10.3390/ijms21218406.
Acknowledgements
Medical writing assistance was provided by Christina McManus, PhD, of Alligent Biopharm Consulting LLC, and funded by Arcutis Biotherapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Arcutis Biotherapeutics, Inc.
Ethics approval and compliance with ethical standards
The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Council for Harmonisation and is registered on ClinicalTrials.gov (NCT03638258). Reviewed and approved by the Aspire Institutional Review Board for US sites, the Western Institutional Review Board for sites in Canada, and local institutional review boards for sites not covered by these central institutional review boards.
Conflict of interest
L. Stein Gold is an investigator for AbbVie, Arcutis, Amgen, Dermavant, Eli Lilly and Company, Leo, Novartis, Ortho Derm, and Pfizer; serves as an advisor for Amgen, Arcutis, BMS, Dermavant, Leo, Novartis, Ortho Derm, Pfizer, and UCB, is a speaker for Amgen, Leo, Ortho Derm, and Pfizer. J. Alonso-Llamazares is an investigator for Arcutis; speaker for Celgene (Amgen), Dermira (Eli Lilly), Eli Lilly and Company, Ortho Derm, and UCB Pharma; and serves on advisory boards for Leo. Z.D. Draelos received grant support from Arcutis Biotherapeutics, Inc. for the conduct of this study. M.J. Gooderham has been a speaker, advisory board member, investigator and/or consultant for AbbVie, Akros, Amgen, Arcutis, BMS, Boehringer-Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Medimmune, Merck, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Valeant/Bausch. S.E. Kempers is an investigator for Arcutis Biotherapeutics, Inc., and serves as a consultant for Foamix and Kinex. L.H. Kircik is an investigator, consultant, speaker, and/or advisory board member for Abbott Laboratories, Acambis, Aclaris, Allergan, Inc., Almirall, Amgen Inc., Anacor Pharmaceuticals, Assos Pharma, Astellas Pharma US, Inc., Asubio, Berlex Laboratories (Bayer HealthCare Pharmaceuticals), Biogen-Idec, Biolife, Biopelle, Boehringer-Ingelheim, Breckinridge Pharma, Celgene, Cellceutix, Centocor, Inc., Cipher, Coherus, Colbar, CollaGenex, Combinatrix, Connetics Corporation, Coria, Dermavant, Dermik Laboratories, Dermira, Dow Pharmaceutical Sciences, Inc., Dusa, Eli Lilly and Company, Embil Pharmaceuticals, EOS, Exeltis, Ferndale Laboratories, Inc., Foamix, Genentech, Inc., GlaxoSmithKline, PLC, Health Point, LTD, Idera, Innocutis, Innovail, Intendis, Isdin, Johnson & Johnson, Laboratory Skin Care Inc., Leo, L'Oreal, 3M, Maruho, Medical International Technologies, Medicis Pharmaceutical Corp., Merck, Merz, Nano Bio, Novartis AG, Noven Pharmaceuticals, Nucryst Pharmaceuticals Corp., Obagi, Onset, OrthoNeutrogena, PediaPharma, PharmaDerm, Pfizer, Promius, PuraCap, QLT, Inc., Quinnova, Quatrix, Serono (Merck Serono International SA), SkinMedica, Inc., Stiefel Laboratories, Inc., Sun Pharma, Taro, TolerRx, Triax, UCB Pharma, Valeant Pharmaceuticals Intl, Warner-Chilcott, XenoPort, and ZAGE. M.G. Lebwohl reports receipt of research funds from AbbVie, Amgen, Arcutis, Boehringer-Ingelheim, Dermavant, Eli Lilly and Company, Incyte, Janssen Research & Development, LLC, Leo Pharma, Ortho Dermatologics, Pfizer, and UCB Pharma and serves as a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., BMD Skincare, Boehringer-Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica. K.A. Papp is an investigator, consultant, speaker, scientific officer or has served on steering committees or advisory boards for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer-Ingelheim, Bristol Myers Squibb, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dice Pharmaceuticals, Dow Pharma, Eli Lilly and Company, Evelo, Galapagos, Galderma, Gilead, GSK, Incyte, Janssen, Kyowa Hakko Kirin, Leo, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda, UCB, and Xencor. D.M. Pariser is an investigator, consultant, and/or advisory board member for Abbott Laboratories, Almirall, Amgen, AOBiome, LLC, Asana Biosciences, LLC, Atacama Therapeutics, Bickel Biotechnology, Biofrontera AG, BMS, Celgene Corporation, Dermavant Sciences, Dermira, Eli Lilly and Company, LEO Pharma, US, Menlo Therapeutics, Merck & Co., Inc, Novartis Pharmaceuticals Corp., Novo Nordisk A/S, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi, Stiefel, a GSK company, TDM SurgiTech, Inc., TheraVida, and Valeant Pharmaceuticals International. D.P. Toth is an investigator and/or consultant for AbbVie, Amgen, Arcutis, Avillion, Bausch Health/Valeant, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly and Company, Galderma, Genentech, GSK, Incyte, Janssen, Leo Pharma, Merck Serono, Medimmune, Novartis, Pfizer, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, and UCB Pharma. G. Yosipovitch has received grant/research support from Bellus Health, Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc., and Sanofi Regeneron and has been a consultant for Bellus Health, Eli Lilly and Company, Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc., Sanofi Regeneron, and Trevi. R. Higham, A. Feng, and D.R. Berk are employees of Arcutis Biotherapeutics, Inc.
Consent to participate
Before enrollment of patients, the study protocol and informed consent form were reviewed and approved by an appropriate Institutional Review Board or Independent Ethics Committee. All patients provided written informed consent before screening.
Consent for publication
Not applicable.
Availability of data and material
Data collected for this study will be made available to others. Proposals for data requests will be reviewed and considered for sharing following approval of the indication. Information about when data availability will begin and end will be provided following approval of the indication.
Author contributions
Authors had full access to all the data in the study and they all take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Feng. Supervision: Higham, Berk.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stein Gold, L., Alonso-Llamazares, J., Draelos, Z.D. et al. Effect of Roflumilast Cream (ARQ-151) on Itch and Itch-Related Sleep Loss in Adults with Chronic Plaque Psoriasis: Patient-Reported Itch Outcomes of a Phase 2b Trial. Am J Clin Dermatol 24, 305–313 (2023). https://doi.org/10.1007/s40257-022-00739-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00739-3