Abstract
Background
Data on treatment outcomes in patients with psoriasis who have skin of color are limited. Brodalumab has shown efficacy in patients with moderate-to-severe plaque psoriasis.
Objective
Our objective was to evaluate the efficacy, safety, and health-related quality of life associated with brodalumab in patients with skin of color participating in two phase III, multicenter, randomized, double-blind, placebo- and active comparator–controlled studies (AMAGINE-2/-3).
Methods
Patients were self-categorized into racial subgroups (black, Asian, or white) or the non-mutually exclusive ethnic subgroup Hispanic/Latino. Patients were randomized to receive brodalumab 210 mg every 2 weeks (Q2W) or ustekinumab (45 mg in patients weighing ≤ 100 kg and 90 mg in patients weighing > 100 kg) for 52 weeks. Skin clearance was monitored using the Psoriasis Area and Severity Index (PASI) and Static Physician’s Global Assessment (sPGA). Treatment-emergent adverse events (TEAEs) were summarized by treatment and racial and ethnic subgroup. Health-related quality of life was assessed using the Dermatology Life Quality Index (DLQI).
Results
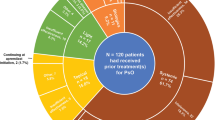
During the 12-week induction phase, 613 patients received ustekinumab (black, n = 20; Asian, n = 24; white, n = 551; Hispanic/Latino, n = 68) and 1236 patients received brodalumab 210 mg Q2W (black, n = 36; Asian, n = 39; white, n = 1116; Hispanic/Latino, n = 132). At week 52, a total of 590 patients received continuous ustekinumab (black, n = 19; Asian, n = 23; white, n = 532; Hispanic/Latino, n = 64) and 339 patients were re-randomized to continue receiving brodalumab 210 mg Q2W (black, n = 10; Asian, n = 7; white, n = 308; Hispanic/Latino, n = 40). Among patients who received brodalumab 210 mg Q2W, skin clearance response rates were similar across racial and ethnic subgroups at week 12 and week 52; rates of 75%, 90%, and 100% improvement in PASI from baseline were also higher, as was sPGA score ≤ 1, than in patients who received ustekinumab across all racial and ethnic subgroups. Rates of TEAEs and ≥ 5-point improvement in DLQI score were similar across racial and ethnic subgroups.
Conclusions
Brodalumab 210 mg Q2W is well tolerated and efficacious across diverse racial and ethnic subgroups in patients with psoriasis, including black, Asian, white, and Hispanic/Latino patients.
Trial Registry
ClinicalTrials.gov identifier NCT01708603 (AMAGINE-2); NCT01708629 (AMAGINE-3).





Similar content being viewed by others
References
Kaufman BP, Alexis AF. Psoriasis in skin of color: Insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19(3):405–23.
Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7(11):16–24.
Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52(1):23–6.
Davis SA, Narahari S, Feldman SR, Huang W, Pichardo-Geisinger RO, McMichael AJ. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):466–73.
McMichael AJ, Vachiramon V, Guzman-Sanchez DA, Camacho F. Psoriasis in African-Americans: a caregivers’ survey. J Drugs Dermatol. 2012;11(4):478–82.
Abrouk M, Lee K, Brodsky M, Nakamura M, Singh R, Zhu TH, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77(1):180–2.
Shah SK, Arthur A, Yang YC, Stevens S, Alexis AF. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10(8):866–72.
Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–86.
Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–9.
Gordon KB, Kimball AB, Chau D, Viswanathan HN, Li J, Revicki DA, et al. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. Br J Dermatol. 2014;170(3):705–15.
Gottlieb AB, Gordon K, Hsu S, Elewski B, Eichenfield LF, Kircik L, et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2018 (Epub ahead of print).
Bushnell DM, Martin ML, McCarrier K, Gordon K, Chiou CF, Huang X, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure to assess psoriasis symptom severity. J Dermatolog Treat. 2013;24(5):356–60.
Langley RGB, Reich K, Papavassilis C, Fox T, Gong Y, Gu Ttner A. Methods for imputing missing efficacy data in clinical trials of biologic psoriasis therapies: implications for interpretations of trial results. J Drugs Dermatol. 2017;16(8):734–41.
Momose M, Asahina A, Umezawa Y, Nakagawa H. Long-term clinical efficacy and safety of secukinumab for Japanese patients with psoriasis: a single-center experience. J Dermatol. 2018;45(3):318–21.
Wu NL, Hsu CJ, Sun FJ, Tsai TF. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44(10):1129–37.
Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–52.
Tsai YC, Tsai TF. A review of clinical trials of biologic agents and small molecules for psoriasis in Asian subjects. G Ital Dermatol Venereol. 2016;151(4):412–31.
Adsit S, Zaldivar ER, Sofen H, Dei-Cas I, Maldonado-Garcia C, Penaranda EO, et al. Secukinumab is efficacious and safe in hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34(6):1327–39.
Gordon KB, Blauvelt A, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2018;178(1):132–9.
Tsai YC, Tsai TF. Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther Adv Musculoskelet Dis. 2017;9(11):277–94.
Woo YR, Cho DH, Park HJ. Molecular mechanisms and management of a cutaneous inflammatory disorder: psoriasis. Int J Mol Sci. 2017;18(12):E2684.
Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–28.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Papp KA, Leonardi CL, Blauvelt A, Reich K, Korman NJ, Ohtsuki M, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3). Br J Dermatol. 2018;178(3):674–81.
Acknowledgements
Editorial assistance was provided under the direction of the authors by MedThink SciCom with support from Brittany Eldridge, PhD and David Boffa, ELS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The AMAGINE-2/-3 studies were supported by Amgen. This analysis was supported by Ortho Dermatologics.
Ethical approval
Study protocols were consistent with the 2008 Declaration of Helsinki and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines for Good Clinical Practice and were approved by individual institutional review boards at each participating study center. All participants signed and personally dated the informed consent form approved by the independent ethics committees and institutional review boards before any study-specific procedures were performed. Additional informed consent was obtained from all individual participants whose photographs appear in this article.
Conflict of interest
Amy McMichael has been an investigator for Allergan, Casseopea, Concert, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, PharmaDerm, Procter & Gamble, and Samumed. Seemal R. Desai has been a consultant to Bausch Health Companies. Andrew F. Alexis has received grants from Dermira, Novartis, and LEO Pharma; consulting fees or honorarium from Novartis, LEO Pharma, and Bausch Health Companies; and speaking fees from LEO Pharma; and has provided writing assistance, medicines, equipment, or administrative support to Bausch Health Companies. Aamir Qureshi is an employee of Ortho Dermatologics and may hold stocks and/or stock options in Bausch Health Companies Pharmaceuticals. Shipra Rastogi may hold stocks and/or stock options in Bausch Health Companies Pharmaceuticals.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McMichael, A., Desai, S.R., Qureshi, A. et al. Efficacy and Safety of Brodalumab in Patients with Moderate-to-Severe Plaque Psoriasis and Skin of Color: Results from the Pooled AMAGINE-2/-3 Randomized Trials. Am J Clin Dermatol 20, 267–276 (2019). https://doi.org/10.1007/s40257-018-0408-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-018-0408-z