Abstract
SnO–V2O5–SiO2 glass anode sample prepared by simple a mechanical milling technique. The amorphous nature of sample identified using with XRD technique. This glass anode has an initial charge capacity of 560 mAhg−1 and discharge capacity of 483 mAhg−1. After 20 charge–discharge cycles, charge and discharge capacities achieved to be 389 and 379 mAhg−1 at 0.1C, respectively. The loss in discharge capacity is up to ~ 45.22% even at high rate 5C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent years, Na-ion battery technology has been proven to be a possible alternative to Li-ion battery technology for large-scale grid storage with further development due to its significant advantages such as cost, natural abundance [1,2,3]. Though Na-ion and Li-ion batteries share similar operating principle, it was shown by various experimental investigations that the respective crystallographic structural functions aiding cationic intercalation are different in case of Li intercalation compounds compared to that of Na-ion intercalation compounds [4, 5]. Moreover, when heavier sodium ions (90 pm) enter into the metal oxide, structural changes occur in the base network, which subsequently limits the performance of the battery [5]. Despite the fact that several crystalline cathode compounds have been developed successfully, slow development of anode materials hampers to realize of room temperature Na-ion batteries [6,7,8]. Unlike Li, metallic sodium is not successful as anode material because of its lower melting temperature (97.7 °C) than Li (180.5 °C) and specific capacity and higher atomic mass. In view of these facts, several groups have been working on variety crystalline anode materials rather than metallic Na; however, each material has their own demerits that include poor electronic conductivity and chemical stability and less specific capacity than its theoretical capacity which are yet to be addressed [9,10,11]. It has been reported that the specific capacities of amorphous glass anode materials are superior than carbon based compounds for both Li- and Na-ion batteries [12, 13].
Among these materials, glass networks mixed with V2O5 paved a way to display interesting structural and Na storage properties in view of the facts that V2O5 exhibits multi-electron transfer at lower voltages, non-zero valency state to convert into its metallic form during intercalation and de-intercalation process [14,15,16,17]. Among these materials, glass networks mixed V2O5 paved a way to display interesting structural and Na storage properties [14,15,16,17]. On the other hand, our group has successfully synthesized, tested, and reported the electrochemical performance of Zn–Ge–Sb glass anode network for use in Na-ion batteries. Here, the initial charge capacity achieved to be 709 mAhg−1 and loss in discharge capacity is up to ~ 18.63% even at high rate 5C which is a significant step to understand intercalation and de-intercalation mechanism of Na-ion batteries [18]. Inspired by the success on glass anodes, it is further required to gather the knowledge on the SnO–V2O5–SiO2 (mol %) glass anode network to meet the desired properties to achieve the demand of high-energy storage in view of the fact V2O5 exhibits low-voltage redox couple of V2+/3+ which we have focused in the present investigation. Furthermore, several experimental investigations have been carried out on variety of compositions of SnO–V2O5–SiO2 (mol %) glass anode network; however, the stoichiometric ratios of SnO, V2O5, and SiO2 compositions have achieved the experimental specific capacity very close to its theoretical capacity which is the objective of our present investigation.
Experimental
SnO–V2O5–SiO2 glass anode prepared by simple mechanical milling method by taking of analytical reagent grade of 33.3 mol % SnO (Sigma-Aldrich, 99.9%), 33.3 mol % V2O5 (Sigma-Aldrich, 99.9%), and 33.3 mol % SiO2 (Sigma-Aldrich, 99.9%). The mechanical milling was carried out using a high-energy planetary ball mill for 50 h to form the homogeneous nanoscaled glass powders at room temperature. Powder-to-ball ratio was taken as 1:10 at 300 rpm and 8 mm tungsten carbide balls were used in milling. XRD technique was used to check the amorphous nature of glass samples [Philips PANalytical X’pert PRO X-ray diffractometer with Cu target (Kα wavelength of 0.154 nm) and Ni filter at 40 kV and 30 mA (2 h range) at room temperature]. Charge/discharge characterizations were carried out using a multichannel battery test unit from Maccor, Inc., model 2000 at a scan rate of 0.1 mV s−1 with a potential window ranging between 0.001 and 2 V.
The working electrode prepared by mixing the active anode glass powder (85% by weight), acetylene black (10% by weight), and polyvinylidene fluoride (PVDF) (5% by weight) with ball miller for 1 h in acetone. Then, the resultant slurry mixture was coated on a copper plate with the help of doctor blade and dried at 70 °C for 24 h. Electrochemical measurements were carried out using Swagelok-type test cells.
Results and discussion
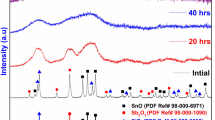
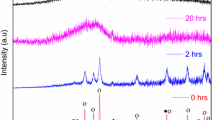
Figure 1 shows the XRD powder patterns of SnO–V2O5–SiO2 glass anode up to 50 h of ball milling. Prior to ball milling (at 0 h), some of crystalline peaks are observed which can well indexed with SnO (ICSD No: 15516) V2O5 (ICSD No: 82152) and SiO2 (ICSD No: 9327). The degree of sharpness and intensity of these crystalline peaks are decreasing slowly with increase in the milling time from 0 to 20 h. Beyond 20 h of ball milling, the intensity of crystalline peaks is slowly decreasing with increase in broadness and almost peaks are disappeared when the ball milling time reaches to 50 h, which confirms that it is amorphous nature (Fig. 1). The structural information of as-prepared glass anode materials obtained via mechanical milling is exactly analogous to the melt quenching technique. The glass transition phenomenon of as synthesized glass anode sample is explored with the help of DTA traces. Figure 2 shows DTA traces related to first five cycles, measured between 373 and 900 K. At first cycle, the crystallization temperature obtained at 754 K due to its exothermic effect which is ascribed due to the formation of VO2 crystals in the glass network. The clear endothermic effect is also observed from the glass transition temperature at 740 K, as depicted in Fig. 2. It is obvious from Fig. 2 that the degree of crystallinity of the present glass anode sample started decreasing from first cycle and shifted to pure amorphous compound after fifth cycle.
Charge and discharge traces of SnO–V2O5–SiO2 glass anode sample from 1st cycle to 20th cycle, measured between 0 and 2 V at 0.1 °C (Fig. 3). It clear from Fig. 3 that the initial charge and discharge capacities evaluated from its abscissa as 560 and 483 mAhg−1, respectively. However, an initial capacity loss measured to be 77 mAhg−1 with 86.2% of columbic efficiency. It is also interesting to note that charge and discharge capacities achieved to be at 389 and 379 mAhg−1 even after 20th cycles with a capacity loss of 10 mAhg−1. Nevertheless, the charge and discharge capacities are retained up to ~ 70 and 80% even for the first 20 cycles (Fig. 3).
In general, variable oxidation states of Sn ions (+2 and +4) will cause for larger volume changes which lowers the initial capacity [19]. However, the presence of VO2 complexes in the SnO–SiO2 glass network will have a super control on agglomeration of Sn particles which will create large number of NBOs (non-bridging oxygens) in the glass network leading to record improved capacity even at 20 cycles.
The discharge cycle performance of SnO–V2O5–SiO2 glass anode sample for various charge and discharge rates (0.1C, 0.5C, 1C, and 5C) is shown in Fig. 4. The loss in discharge capacity is measured to be ~ 8.71, 11.82, 11.24, and 10.60% at 0.1C, 0.5C, 1C, and 5C, respectively (Fig. 4). Moreover, the loss in discharge capacity is recorded up to ~ 45.22% even in high rate at 5C for the initial discharge. On the other hand, the highest columbic efficiency ~ 97.43% is recorded at 0.1C rate (Fig. 3) which is a significant trend of our present glass anode sample for efficient use in the storage devices.
Conclusion
SnO–V2O5–SiO2 glass anode system was synthesized by mechanical milling technique. The presence endothermic effect in the DTA curves clearly suggests that the present anode sample is perfectly in amorphous state after third and fifth cycles. Initial charge and discharge capacities evaluated to be 560 and 483 mAhg−1, respectively, and will be retained up to ~ 70 and 80% even for the first 20 cycles.
References
Bruce, P.G., Scrosati, B., Tarascon, J.M.: Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47(16), 2930–2946 (2008)
Idota, Y., Kubota, T., Matsufuji, A., Maekawa, Y., Miyasaka, T.: Tin-based amorphous oxide: a high-capacity lithium-ion-storage material. Science 276(5317), 1395–1397 (1997)
Goodenough, J.B., Kim, Y.: Challenges for rechargeable Li batteries. Chem. Mater. 22(3), 587–603 (2009)
Sawicki, M., Shaw, L.L.: Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Advances 5(65), 53129–53154 (2015)
Massé, R.C., Liu, C., Li, Y., Mai, L., Cao, G.: Energy storage through intercalation reactions: electrodes for rechargeable batteries. Nat. Sci. Rev. 4(1), 26–53 (2017)
Kim, S.W., Seo, D.H., Ma, X., Ceder, G., Kang, K.: Electrode materials for rechargeable Sodium-Ion batteries: potential alternatives to current Lithium-Ion batteries. Adv. Energy Mater 2, 710–721 (2012)
Morinaga, K., Fujino, S.: Preparation and properties of SnO–SnCl2–P2O5 glass. J. Non Cryst. Solids 282(1), 118–124 (2001)
Hwang, J.Y., Myung, S.T., Sun, Y.K.: Sodium-ion batteries: present and future. Chem. Soc. Rev. 46(12), 3529–3614 (2017)
Lu, Y.C., Ma, C., Alvarado, J., Kidera, T., Dimov, N., Meng, Y.S., Okada, S.: Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sour. 284, 287–295 (2015)
Kim, Y., Ha, K.H., Oh, S.M., Lee, K.T.: High-capacity anode materials for Sodium-Ion Batteries. Chem. Eur. J. 20(38), 11980–11992 (2014)
Kang, H., Liu, Y., Cao, K., Zhao, Y., Jiao, L., Wang, Y., Yuan, H.: Update on anode materials for Na-ion batteries. J. Mater. Chem. A 3(35), 17899–17913 (2015)
Yamauchi, H., Park, G., Nagakane, T., Honma, T., Komatsu, T., Sakai, T., Sakamoto, A.: Performance of lithium-ion battery with tin-phosphate glass anode and its characteristics. J. Electrochem. Soc. 160(10), A1725–A1730 (2013)
Hayashi, A., Konishi, T., Tadanaga, K., Minami, T., Tatsumisago, M.: All-solid-state rechargeable lithium batteries using SnX–P2X5 (X = S and O) amorphous negative electrodes. Res. Chem. Intermed. 32(5), 497–506 (2006)
Sakurai, Y., Yamaki, J.I.: Correlation between microstructure and electrochemical behavior of amorphous V2O5–P2O5 in Lithium cells. J. Electrochem. Soc. 135(4), 791–796 (1988)
Sakurai, Y., Okada, S., Yamaki, J., Okada, T.: Electrochemical behaviour of amorphous V2O5–(P2O5) cathodes for lithium secondary batteries. J. Power Sour. 20(3–4), 173–177 (1987)
Leech, A.C., Owen, J.R., Steele, B.C.H.: Lithium insertion into a vanadium boron oxide glass. Solid State Ionics 9–10(1), 645–648 (1983)
Levy, M., Rousseau, F., Duclot, M.: Electrochemical properties of glasses in the TeO2−V2O5 system. Solid State Ionics 28, 736–738 (1988)
Ravuri, B.R., Suman, G., Srinivasa Rao, C.: Zn–Ge–Sb glass composite mixed with Ba2+ ions: a high capacity anode material for Na-ion batteries. Appl. Nanosci. (2018). https://doi.org/10.1007/s13204-018-0822-9
Honma, T., Togashi, T., Kondo, H., Komatsu, T., Yamauchi, H., Sakamoto, A., Sakai, T.: Tin-phosphate glass anode for sodium ion batteries. Apl Materials 1(5), 052101 (2013)
Acknowledgements
This study was supported by the Grant-in-Aid for scientific research from the Naval Research Board, DRDO, Govt. of India (Grant No: NRB-311/MAT/13-14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gandi, S., Chinta, S.R., Katari, N.K. et al. Electrochemical performance of SnO–V2O5–SiO2 glass anode for Na-ion batteries. Mater Renew Sustain Energy 7, 22 (2018). https://doi.org/10.1007/s40243-018-0129-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-018-0129-5