Abstract
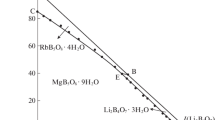
The metastable phase equilibria of the Li+, Mg2+//SO4 2–, borate-H2O system at 273.15 K were studied using isothermal evaporation method. The dry-salt phase diagram, water-phase diagram and the physicochemical property diagrams of the system were plotted with the metastable solubility values and physicochemical properties corresponding to density, refractive index, pH value and conductivity. The dry-salt diagram was composed of four crystallizing zones[lithium sulfate hydrate(Li2SO4·H2O), epsomite(MgSO4·7H2O), lithium metaborate octahydrate(LiBO2·8H2O), and hungchaoite(MgB4O7·9H2O)], five univariant curves and two invariant points (Li2SO4·H2O+MgSO4·7H2O+MgB4O7·9H2O and Li2SO4·H2O+LiBO2·8H2O+MgB4O7·9H2O). Li2B4O7 converted into LiBO2 in solution. Comparing the metastable phase diagram at 273.15 K and stable phase diagram at 298.15 K for the system, the crystallized area of Li2SO4·H2O and MgSO4·7H2O became large, whereas, the other phase regions became small. The J(H2O) changes regularly with increasing J(SO4 2‒), and the physicochemical properties change regularly with the concentration of B4O7 2‒ increasing.
Similar content being viewed by others
References
Zheng X. Y., Tang Y., Xu C., Tibet Saline Lake, Chinese Science Press, Beijing, 1988
Sun B., Song P. S., Du X. H., J. Salt Lake Res., 1994, 2(4), 26
Li Z. W., Qian Z. P., Chen X., Dang Y. L., Yang Q. C., Chem. J. Chinese Universities, 2015, 36(9), 1759
Huang Y., Zou F., Ni S. J., Sang S. H., Chem. Res. Chinese Universi-ties, 2011, 27(3), 482
Li L., Zhang S. S., Liu Y. H., Guo Y. F., Deng T. L., Chem. J. Chinese Universities, 2016, 37(2), 349
Song P. S., Sun B., Zeng D. W., Pure Appl. Chem., 2013, 85(11), 2097
Meng L.Z., Li D., Braz. J. Chem. Eng., 2014, 31(1), 251
Deng T. L., Meng L. Z., Sun B., J. Chem. Eng. Data, 2008, 53(3), 704
Gao D. L., Guo Y. F., Yu X. P., Wang S. Q., Deng T. L., J. Chem. Eng. Data, 2015, 60(9), 2594
Song P. S., Fu H. A., J. Inorg. Chem., 1991, 7(3), 344
Jing Y., Sea-lake Salt and Chemical Industry, 2000, 29(2), 24
Zhai Z. X., Analytical Methods of Brines and Salts, 2nd Ed., Chinese Science Press, Beijing, 1988
Wang S. Q., Gao J., Yu X., Sun B., Deng T. L., J. Salt Lake Res., 2007, 15, 44
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(Nos.21406104, U1407113, U1507112 and U1607123), the China Postdoctoral Science Foundation(No.2015M581303), the Yangtze Scholar and Innovative Research Team in Chinese University(No.IRT_17R81) and the Fund of Key Laboratory of Salt Lake Resources and Chemistry, Qinghai Institute of Salt Lake, Chinese Academy of Sciences in China (No.KLSLRC-KF-13-HX-2).
Rights and permissions
About this article
Cite this article
Meng, L., Guo, Y., Li, D. et al. Solid and liquid metastable phase equilibria in the aqueous quaternary system Li+, Mg2+//SO4 2–, borate-H2O at 273.15 K. Chem. Res. Chin. Univ. 33, 655–659 (2017). https://doi.org/10.1007/s40242-017-6404-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-017-6404-7