Abstract
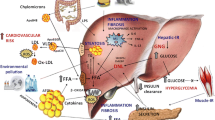
Energy imbalance resulting from high calorie food intake and insufficient metabolic activity leads to increased body mass index (BMI) and sets the stage for metabolic derangement influencing lipid and carbohydrate metabolism and ultimately leading to insulin resistance, dyslipidemia, and type 2 diabetes. 70% of cardiovascular disease (CVD) deaths occur in patients with diabetes. Environment-induced physiological perturbations trigger epigenetic changes through chromatin modification and leads to type 2 diabetes and progression of nonalcoholic fatty liver disease (NAFLD) and CVD. Thus, in terms of disease progression and pathogenesis, energy homeostasis, metabolic dysregulation, diabetes, fatty liver, and CVD are interlinked. Since advanced glycation end products (AGEs) and low-grade inflammation in type 2 diabetes play definitive roles in the pathogenesis of liver and vascular diseases, a natural checkpoint to prevent diabetes and associated complications appears to be the identification and management of prediabetes together with weight management, since 70% of prediabetic individuals develop diabetes during their life time, and every kg of weight increase is associated with up to 9% increase in diabetes risk. A good proportion of diabetes and obesity population have fatty liver that progresses to non-alcoholic steatohepatitis (NASH) and cirrhosis, and increased risk of hepatocellular carcinoma. Diabetes and NASH both have elevated oxidative stress, impaired cholesterol elimination, and increased inflammation that leads to CVD risk. This review addresses life-style-induced metabolic pathway derangement and how it contributes to epigenetic changes, type 2 diabetes and NASH progression, which collectively lead to increased risk of CVD.







Similar content being viewed by others
Abbreviations
- VLDL:
-
very low-density lipoprotein
- LDL:
-
low-density lipoprotein
- HDL:
-
high-density lipoprotein
- CVD:
-
cardiovascular disease
- NAFLD:
-
non-alcoholic fatty liver disease
- AGE:
-
advanced glycation end-products
- NASH:
-
non-alcoholic steatohepatitis
- MetS:
-
metabolic syndrome
- apoA-I:
-
apolipoprotein A-I
- BMI:
-
body mass index
- miR:
-
micro RNA
- COPD:
-
chronic obstructive pulmonary disease
- HAT:
-
histone deacetylase
- HDAC:
-
histone acetyl transferase
- T2DM:
-
type 2 diabetes mellitus
- VSMC:
-
Vascular smooth muscle cells
- MCP1:
-
monocyte chemoattractant protein 1
- LSD-1:
-
lysine-specific demethylase
- VCAM1:
-
vascular cell adhesion molecule 1
- NF-κB:
-
nuclear factor kappa B
- Cox2:
-
cyclooxygenase 2
- TNF-α:
-
tumor necrosis factor-α
- FXR:
-
farnesoid X receptor
- TRL:
-
triglyceride-rich lipoproteins
- RCT:
-
reverse cholesterol transport
- SR-BI:
-
scavenger receptor class B type 1
- ABCA1:
-
ATP binding cassette transporter 1
- HbA1c:
-
hemoglobin A1c
- RAGE:
-
receptor for advanced glycation end-products
References
Cavan DHJ, Linnenkamp U, Makaroff L, Magliano D, Ogurtrstova K, Shaw J. Diabetes and cardiovascular disease. International Diabetes Federation 2016;1–12. http://www.idforg/cvd. Accessed 9 Aug 2018.
Feingold KR, Grunfeld C. Obesity and dyslipidemia. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc., 2000. Acessed 10 April 2018.
Srivastava RA, Srivastava N. Search for obesity drugs: targeting central and peripheral pathways. Curr Med Chem Immunol Endocr Metab Agents. 2004;4:75–90.
Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens, Greece). 2018;17:299–313.
Davidson MH, Bays HE, Stein E, Maki KC, Shalwitz RA, Doyle R. Effects of fenofibrate on atherogenic dyslipidemia in hypertriglyceridemic subjects. Clin Cardiol. 2006;29:268–73.
Riserus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–9.
Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16:6–11.
Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:171–83.
Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–63.
Hirano T. Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb. 2018;25:771–82.
Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62.
Oorni K, Lehti S, Sjovall P, Kovanen PT. Triglyceride-rich lipoproteins as a source of proinflammatory lipids in the arterial wall. Curr Med Chem. 2018
Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta. 2012;1821:867–75.
Al-Zakwani I, Sulaiman K, Al-Rasadi K, Mikhailidis DP. Prevalence of low high-density lipoprotein cholesterol (HDL-C) as a marker of residual cardiovascular risk among acute coronary syndrome patients from Oman. Curr Med Res Opin. 2011;27:879–85.
Sniderman AD, Faraj M. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr Opin Lipidol. 2007;18:633–7.
Pietzsch J, Julius U, Nitzsche S, Hanefeld M. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47:1928–34.
Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–5.
Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res. 2012;53:2490–514.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–27.
Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. Jama. 2015;314:1021–9.
Yates T, Khunti K, Troughton J, Davies M. The role of physical activity in the management of type 2 diabetes mellitus. Postgrad Med J. 2009;85:129–33.
Eckel RH. CVD health factors and CVD risk factors: state of the science, emerging priorities part 2: obesity prevention. Ethn Dis. 2012;22:S1–36–40.
Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401.
Mooradian AD, Haas MJ, Wehmeier KR, Wong NC. Obesity-related changes in high-density lipoprotein metabolism. Obesity (Silver Spring). 2008;16:1152–60.
Ooi EM, Watts GF, Farvid MS, et al. High-density lipoprotein apolipoprotein A-I kinetics in obesity. Obes Res. 2005;13:1008–16.
Basciano H, Miller AE, Naples M, Baker C, Kohen R, Xu E, et al. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Phys Endocrinol Metab. 2009;297:E462–73.
Srivastava RA, He S. Anti-hyperlipidemic and insulin sensitizing activities of fenofibrate reduces aortic lipid deposition in hyperlipidemic Golden Syrian hamster. Mol Cell Biochem. 2010;345:197–206.
Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24:198–206.
Anjana RM, Pradeepa R, Deepa M, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7.
Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India--the Chennai urban rural epidemiology study (CURES-17). Diabetologia. 2006;49:1175–8.
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. Jama. 2013;310:948–59.
Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. Jama. 2017;317:2515–23.
Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8.
Chambon P. Determination of the organization of coding and intervening sequences in the chicken ovalbumin split gene. Differentiation. 1979;13:43–4.
Crick F. Split genes and RNA splicing. Science (New York, NY). 1979;204:264–71.
Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. 2016;65:1895–905.
Khullar M, Cheema BS, Raut SK. Emerging evidence of epigenetic modifications in vascular complication of diabetes. Front Endocrinol. 2017;8:237.
Clouaire T, Stancheva I. Methyl-CpG binding proteins: specialized transcriptional repressors or structural components of chromatin? Cell Mol Life Sc. 2008;65:1509–22.
Patra SK, Patra A, Rizzi F, Ghosh TC, Bettuzzi S. Demethylation of (Cytosine-5-C-methyl) DNA and regulation of transcription in the epigenetic pathways of cancer development. Cancer Metastasis Rev. 2008;27:315–34.
Marmorstein R, Trievel RC. Histone modifying enzymes: structures, mechanisms, and specificities. Biochim Biophys Acta. 2009;1789:58–68.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705.
Avvakumov N, Cote J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–407.
Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42.
Jayaraman S. Epigenetic mechanisms of metabolic memory in diabetes. Circ Res. 2012;110:1039–41.
El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17.
Wren JD, Garner HR. Data-mining analysis suggests an epigenetic pathogenesis for type 2 diabetes. J Biomed Biotechnol. 2005;2005:104–12.
Parrizas M, Maestro MA, Boj SF, Paniagua A, Casamitjana R, Gomis R, et al. Hepatic nuclear factor 1-alpha directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol Cell Biol. 2001;21:3234–43.
Francis J, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. J Biol Chem. 2005;280:36244–53.
Mosley AL, Ozcan S. The pancreatic duodenal homeobox-1 protein (Pdx-1) interacts with histone deacetylases Hdac-1 and Hdac-2 on low levels of glucose. J Biol Chem. 2004;279:54241–7.
Ozcan S. Minireview: microRNA function in pancreatic beta cells. Mol Endocrinol. 2014;28:1922–33.
Mosley AL, Ozcan S. Glucose regulates insulin gene transcription by hyperacetylation of histone h4. J Biol Chem. 2003;278:19660–6.
Chakrabarti SK, Francis J, Ziesmann SM, Garmey JC, Mirmira RG. Covalent histone modifications underlie the developmental regulation of insulin gene transcription in pancreatic beta cells. J Biol Chem. 2003;278:23617–23.
Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–52.
Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103:615–23.
Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–81.
Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–7.
Jones Buie JN, Goodwin AJ, Cook JA, Halushka PV, Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–81.
Aguilar-Olivos NE, Oria-Hernandez J, Ponciano-Rodriguez G, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. The role of epigenetics in the progression of non-alcoholic fatty liver disease. Mini Rev Med Chem. 2015;15:1187–94.
Srivastava RAK. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol Cell Biochem. 2018;440:167–87.
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–90.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289–97.
Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579–88.
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44.
Datz C, Muller E, Aigner E. Iron overload and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:173–83.
Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin N Am. 2016;45:639–52.
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42.
Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine. 2017;96:e8179.
Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84.
Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metab Clin Exp. 2016;65:1096–108.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85.
Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34.
Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46.
Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–92.
Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–7.
Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–80.
Leung C, Herath CB, Jia Z, Andrikopoulos S, Brown BE, Davies MJ, et al. Dietary advanced glycation end-products aggravate non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8026–40.
Sayej WN, Knight Iii PR, Guo WA, et al. Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. Biomed Res Int. 2016;2016:7867852.
Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112–9.
Sebekova K, Kupcova V, Schinzel R, Heidland A. Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis - amelioration by liver transplantation. J Hepatol. 2002;36:66–71.
Reeves HL, Zaki MY, Day CP. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234–45.
Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–88.
Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–8.
Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–24.
Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–8.
Poulsen MM, Larsen JO, Hamilton-Dutoit S, Clasen BF, Jessen N, Paulsen SK, et al. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res. 2012;32:701–8.
Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–61.
Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–92.
Choi YJ, Kim DH, Kim SJ, Kim J, Jeong SI, Chung CH, et al. Decursin attenuates hepatic fibrogenesis through interrupting TGF-beta-mediated NAD(P)H oxidase activation and Smad signaling in vivo and in vitro. Life Sci. 2014;108:94–103.
Tarling EJ, Clifford BL, Cheng J, Morand P, Cheng A, Lester E, et al. RNA-binding protein ZFP36L1 maintains posttranscriptional regulation of bile acid metabolism. J Clin Invest. 2017;127:3741–54.
Thayer TEMT, Shakartzi H, Wunderer F, Perrien D, Farber-Eger E, Burke M, et al. Inhibition of bone morphogenetic protein (BMP) signaling mitigates nonalcoholic fatty liver disease (NAFLD) and identification of an activating BMP receptor polymorphism associated with NAFLD. Circulation. 2017;136:A16421.
Baffy G. MicroRNAs in nonalcoholic fatty liver disease. J Clin Med. 2015;4:1977–88.
He Z, Hu C, Jia W. miRNAs in non-alcoholic fatty liver disease. Front Med. 2016;10:389–96.
Gallego-Duran R, Romero-Gomez M. Epigenetic mechanisms in non-alcoholic fatty liver disease: an emerging field. World J Hepatol. 2015;7:2497–502.
Zhang M, Sun W, Zhou M, Tang Y. MicroRNA-27a regulates hepatic lipid metabolism and alleviates NAFLD via repressing FAS and SCD1. Sci Rep. 2017;7:14493.
Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–68.
Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond). 2011;121:43–55.
Forbes JM, Yee LT, Thallas V, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–23.
Win MT, Yamamoto Y, Munesue S, et al. Regulation of RAGE for attenuating progression of diabetic vascular complications. Exp Diabetes Res. 2012;2012:894605.
Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–9.
Saremi A, Howell S, Schwenke DC, Bahn G, Beisswenger PJ, Reaven PD. Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow-up study. Diabetes Care. 2017;40:591–8.
Sun B, Li X, Liu X, Ge X, Lu Q, Zhao X, et al. Association between carotid plaque characteristics and acute cerebral infarction determined by MRI in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16:111.
Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–8.
Srivastava R, Cefalu, AB, Davide, A, Averna MR. A Combination of Metformin, Quercetin, and Curcumin Restores HDL Function and Improves Atherosclerosis Burden in LDLr−/−/ob.ob leptin−/− and LDLr−/− Mice by attenuating Inseulin Resistance, Hyperglycemia, and Low-grade Inflammation ATVB Scientific Session 2013:Abstract.
Srivastava RAK. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol Cell Biochem. 2018;440:167–187.
Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC study. Atherosclerosis risk in communities study. Circulation. 1995;91:1432–43.
Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–80.
Romero MJ, Platt DH, Tawfik HE, Labazi M, el-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102.
Tkac I, Kimball BP, Lewis G, Uffelman K, Steiner G. The severity of coronary atherosclerosis in type 2 diabetes mellitus is related to the number of circulating triglyceride-rich lipoprotein particles. Arterioscler Thromb Vasc Biol. 1997;17:3633–8.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605.
Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346:161–70.
Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–55.
Srivastava RA, Srivastava N. High density lipoprotein, apolipoprotein A-I, and coronary artery disease. Mol Cell Biochem. 2000;209:131–44.
Gatti A, Maranghi M, Bacci S, Carallo C, Gnasso A, Mandosi E, et al. Poor glycemic control is an independent risk factor for low HDL cholesterol in patients with type 2 diabetes. Diabetes Care. 2009;32:1550–2.
Hoang A, Murphy AJ, Coughlan MT, Thomas MC, Forbes JM, O’Brien R, et al. Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia. 2007;50:1770–9.
Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60:2617–23.
Ohgami N, Miyazaki A, Sakai M, Kuniyasu A, Nakayama H, Horiuchi S. Advanced glycation end products (AGE) inhibit scavenger receptor class B type I-mediated reverse cholesterol transport: a new crossroad of AGE to cholesterol metabolism. J Atheroscler Thromb. 2003;10:1–6.
Srivastava N, Cefalu AB, Averna M, Srivastava RAK. Lack of correlation of plasma HDL with fecal cholesterol and plasma cholesterol efflux capacity suggests importance of HDL functionality in attenuation of atherosclerosis. Front Physiol. 2018;9:1222.
Low H, Hoang A, Forbes J, Thomas M, Lyons JG, Nestel P, et al. Advanced glycation end-products (AGEs) and functionality of reverse cholesterol transport in patients with type 2 diabetes and in mouse models. Diabetologia. 2012;55:2513–21.
Passarelli M, Tang C, McDonald TO, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–205.
McGillicuddy FC, de la Llera Moya M, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45.
Apro J, Tietge UJ, Dikkers A, Parini P, Angelin B, Rudling M. Impaired cholesterol efflux capacity of high-density lipoprotein isolated from interstitial fluid in type 2 diabetes mellitus-brief report. Arterioscler Thromb Vasc Biol. 2016;36:787–91.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, R.A.K. Life-style-induced metabolic derangement and epigenetic changes promote diabetes and oxidative stress leading to NASH and atherosclerosis severity. J Diabetes Metab Disord 17, 381–391 (2018). https://doi.org/10.1007/s40200-018-0378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-018-0378-y