Abstract
Purpose
To prepare freeze-dried bupivacaine lipospheres intended for topical application in burn injuries. The aim was improving the storage stability and developing a prolonged release pattern to tackle the adverse reactions resulting from the frequent administration of bupivacaine.
Methods
The lipospheres were prepared by hot-melt dispersion method employing bupivacaine base at 1.5 and 3%w/w, tristearin 6% w/w as the core while dipalmitoyl phosphatidylcholine (DPPC) and soy phosphatidylcholine (SPC) as the coat at 0.75, 1.5 and 3% w/w. The lotion was then freeze-dried and cryoprotected by sucrose 3% w/w. Evaluation was carried out through loading and release analysis, storage study, particle characterization including morphology, zeta potential and particle size as well as anti-microbial assessment.
Results
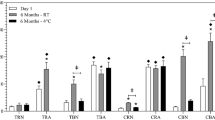
The highest loading, (87.6 ± 0.1%), was achieved using bupivacaine 3% and SPC 0.75%. After 6 months of storage at 4 ͦC, the loading in the lotion and the freeze-dried samples were 17.4 ± 0.2 and 87.2 ± 0.3%, respectively. In vitro dissolution test demonstrated 94.5% and 95% of bupivacaine release from lotion and freeze-dried samples, after 24 h. The respective zeta potential of -1.30 and 26 mV was recorded for lotion and solid-state bupivacaine. Micromeritic evaluation of freeze-dried powder exhibited particle size of 35.23 ± 2.02 μm and highly-wrinkled-irregular morphology without detectable needle structures related to drug free crystals. The powder had rapid reconstitution property and antibacterial activity.
Conclusion
Freeze- drying holds a promising potential to improve the storage stability of bupivacaine lipospheres with well- preserved release pattern and particle properties for further topical application.
Graphical abstract



Similar content being viewed by others
Data availability
Not applicable.
References
Myers R, et al. Sedation and Analgesia for Dressing Change: a survey of American Burn Association Burn Centers. J Burn Care Res. 2017;38(1):e48–54.
Hernandez JL, et al. Use of continuous local anesthetic infusion in the management of postoperative split-thickness skin graft donor site pain. J Burn Care Res. 2013;34(4):e257-262.
Jellish WS, et al. Effect of topical local anesthetic application to skin harvest sites for pain management in burn patients undergoing skin-grafting procedures. Ann Surg. 1999;229(1):115–20.
Desai C, et al. Effectiveness of a topical local anaesthetic spray as analgesia for dressing changes: a double-blinded randomised pilot trial comparing an emulsion with an aqueous lidocaine formulation. Burns. 2014;40(1):106–12.
Toongsuwan S, et al. Formulation and characterization of bupivacaine lipospheres. Int J Pharm. 2004;280(1):57–65.
Malinovsky JM, et al. Motor and blood pressure effects of epidural sustained-release bupivacaine from Polymer microspheres: a dose-response study in rabbits. Anesth Analg. 1995;81(3):519–24.
Kopacz DJ et al. A model to evaluate the pharmacokinetic and pharmacodynamic variables of extended-release products using in vivo tissue microdialysis in humans: bupivacaine-loaded microcapsules. Anesth Analg. 2003;97(1):124 − 31. table of contents.
Masters DB, Domb AJ. Liposphere local anesthetic timed-release for perineural site application. Pharm Res. 1998;15(7):1038–45.
Chime SA, et al. Sustained-release diclofenac potassium-loaded solid lipid microparticle based on solidified reverse micellar solution: in vitro and in vivo evaluation. J Microencapsul. 2013;30(4):335–45.
Audu M, Achile PA, Amaechi AA. Phospholipon 90G based SLMs loaded with ibuprofen: an oral antiinflammatory and gastrointestinal sparing evaluation in rats. Pakistan J Zool. 2012;44:1657–64.
Vase H, Nemattalab M, Rohani M, Hesari Z. Comparison of chitosan and SLN nano-delivery systems for antibacterial effect of tea tree (Melaleuca alternifolia) oil against P. aeruginosa and S. aureus. Lett Appl Microbiol. 2023;76(11). https://doi.org/10.1093/lambio/ovad130.
Souto EB, et al. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278(1):71–7.
Nasr M, et al. Lipospheres as carriers for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. AAPS PharmSciTech. 2008;9(1):154–62.
El-Nesr OH, Yahiya SA, El-Gazayerly ON. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm J. 2010;18(4):217–24.
Varshosaz J, Eskandari S, Tabbakhian M. Freeze-drying of nanostructure lipid carriers by different carbohydrate polymers used as cryoprotectants. Carbohydr Polym. 2012;88(4):1157–63.
Chime SA, Gugu TH. Effect of lyophilization on the physicochemical and physicotechnical properties of aspirin-loaded lipospheres. Innovare J Sci. 2014;2(3):4–8.
Lee MK, et al. Cryoprotectants for freeze drying of drug nano-suspensions: effect of freezing rate. J Pharm Sci. 2009;98(12):4808–17.
Leng D, et al. Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharm Res. 2018;35(12):247.
Faghihi H, et al. The effect of freeze-dried antibody concentrations on its stability in the presence of trehalose and hydroxypropyl-β-cyclodextrin: a box–behnken statistical design. Pharm Dev Technol. 2017;22(6):724–32.
Prajapati L, et al. Lipospheres: recent advances in various drug delivery system. Int J Pharm Technol. 2013;5:2446–64.
Tomar Y, Maheshwari S, Gorantla S, Singhvi G. Curcumin loaded liquid crystalline nanoparticles for enhanced topical application: Design, characterization, ex vivo and dermatokinetic evaluation. J Drug Deliv Sci Technol. 2024;92:105391. https://www.sciencedirect.com/science/article/pii/S1773224724000595. https://doi.org/10.1016/j.jddst.2024.105391.
Rohani M, Nemattalab M, Hedayati M, Ghasemi S, Hesari Z. Comparison of chitosan and SLN nano-delivery systems for antibacterial effect of cinnamon (Cinnamomum verum) oil against MDR K pneumoniae and E coli. Phys Scr. 2023;98(10):105002. https://doi.org/10.1088/1402-4896/acf3a5.
Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv Pharm Bull. 2013;3(1):25–9.
Valjakka-Koskela R, et al. Enhancement of percutaneous absorption of naproxen by phospholipids. Int J Pharm. 1998;175(2):225–30.
Li J, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10(2):81–98.
Momoh MA, Kenechukwu FC, Attama AA. Formulation and evaluation of novel solid lipid microparticles as a sustained release system for the delivery of metformin hydrochloride. Drug Deliv. 2013;20(3–4):102–11.
Kim B-D, Na K, Choi H-K. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur J Pharm Sci: Official J Eur Federation Pharm Sci. 2005;24:2–3.
Taylor KMG, Morris RM. Thermal analysis of phase transition behaviour in liposomes. Thermochim Acta. 1995;248:289–301.
Iscan Y, et al. DEET-loaded solid lipid particles for skin delivery: in vitro release and skin permeation characteristics in different vehicles. J Microencapsul. 2006;23(3):315–27.
Vighi E, et al. Re-dispersible cationic solid lipid nanoparticles (SLNs) freeze-dried without cryoprotectors: characterization and ability to bind the pEGFP-plasmid. Eur J Pharm Biopharm. 2007;67(2):320–8.
Schreier H, Levy M, Mihalko P. Sustained release of liposome-encapsulated gentamicin and fate of phospholipid following intramuscular injection in mice. J Controlled Release. 1987;5(2):187–92.
Adler DMT, Damborg P, Verwilghen DR. The antimicrobial activity of bupivacaine, lidocaine and mepivacaine against equine pathogens: an investigation of 40 bacterial isolates. Vet J. 2017;223:27–31.
Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt). 2008;9(2):205–13.
Kesici S, Demırci M, Kesici U. Antimicrobial effects of fentanyl and bupivacaine]. Braz J Anesthesiol. 2020;70(4):357–63.
Casillas-Vargas G, et al. Antibacterial fatty acids: an update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog Lipid Res. 2021;82: 101093.
Yoon BK, Jackman JA, Valle-González ER, Cho NJ. Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int J Mol Sci. 2018;19(4). https://doi.org/10.3390/ijms19041114.
Nemati S, et al. Formulation of neem oil-loaded solid lipid nanoparticles and evaluation of its anti-toxoplasma activity. BMC Complement Med Ther. 2022;22(1):122.
Funding
There were not any financial supports.
Author information
Authors and Affiliations
Contributions
This study was a part of a Pharm D thesis of SL who did all the practical experiments and drafted the article. Authors (HF, AJ, and HM) contributed to editing the manuscript. HM was the supervisor of anti-microbial examination. HF supervised all the steps of the thesis. All authors read and approved the final copy of the text.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labanian, S., Faghihi, H., Montazeri, H. et al. Freeze-drying of bupivacaine lipospheres: preparation, characterization, and evaluation of anti-microbial properties. DARU J Pharm Sci 32, 207–214 (2024). https://doi.org/10.1007/s40199-024-00506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-024-00506-1