Abstract
Purpose
Type 2 diabetes mellitus (T2DM) is the subject of numerous randomized controlled trials (RCTs). The validity of RCTs may be threatened by attrition bias due to the discontinuation of the study. The aim of this systematic review is to evaluate the reasons of patient’s withdrawal from these RCTs.
Methods
A systematic literature search on PubMed, Cochrane Library, Web of Science, and Scopus databases was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. The aim was to obtain all relevant blinded RCTs published before January 2017 in which the effectiveness of synthetic drugs, vitamins/minerals were compared to that of placebo or active control in T2DM. The quality of RCTs was assessed using the Jadad score. The frequency of withdrawal reasons was presented based on treatments with placebo/active control, national/international level of the studies, and publication year. Meta-analysis was not performed due to the heterogeneity.
Results
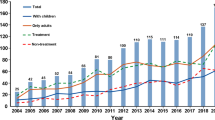
Overall, 1368 articles comprising of 640,780 subjects were included. In the majority of the RCTs (75.0%), the intervention and the placebo arms were compared. Most of the included studies (96%) were classified in the high-quality category (Jadad score≥3). The highest proportion of reported withdrawal cases was found in international studies, national RCTs conducted in Japan, and RCTs published in 2011. The withdrawal reasons were reported for 91,669 (63.75%) of the total 143,794 participants who had withdrawn from these studies. The main reported reasons were “adverse effects” (24.04%), “withdraw consent” (16.10%), and “missing data” (11.08%). Variations in the reported withdrawal reasons were based on the country or published year. RCTs with triple blinded design as well as those in which anti-hyperlipidemia and anti-obesity medications were applied, showed significantly higher probability of reported the withdrawal.
Conclusion
High proportion of reported discontinuation in blinded RCTs on patients with T2DM was related to drug adverse effects. Overall, the total number and reason of drop out were unsatisfactory.


Similar content being viewed by others
References
WHO. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation, Part 1: diagnosis and classification of diabetes mellitus. World Health Organization 1999, Geneva.
Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–73.
Tabatabaei-Malazy O, Larijani B, Abdollahi M. Targeting metabolic disorders by natural products. J Diabetes Metab Disord. 2015;14:57. https://doi.org/10.1186/s40200-015-0184-8.
IDF Diabetes atlas, 9th edition 2019. Available at https://www.diabetesatlas.org. .
Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Drugs for the treatment of pediatric type 2 diabetes mellitus and related co-morbidities. Expert Opin Pharmacother. 2016;17(18):2449–60. https://doi.org/10.1080/14656566.2016.1258057.
Tootee A, Esfahani EN, Larijani B. Diabetes management during Ramadan amid Covid-19 pandemic. Daru. 2020;28:1–4. https://doi.org/10.1007/s40199-020-00357-6.
DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–9.
Kabisch M, Ruckes C, Seibert-Grafe M, Blettner M. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011;108(39):663–8.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Manchikanti L, Hirsch JA, Smith HS. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part 2: randomized controlled trials. Pain Physician. 2008;11(6):717–73.
Stang A. Randomized controlled trials-an indispensible part of clinical research. Dtsch Arztebl Int. 2011;108(39):661–2.
Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–6.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Pandis N, Chung B, Scherer RW, Elbourne D, Altman DG. CONSORT 2010 statement: extension checklist for reporting within person randomised trials. BMJ. 2017;357:j2835. https://doi.org/10.1136/bmj.j2835.
Hasanshiri F, Moosavi S, Atashzadeh-Shoorideh F. Self-care programs in diabetic patients: quality of methodological report in randomized controlled trials published in Iranian journals between 2010-2016. Iranian Journal of Endocrinology and Metabolism. 2017;18(6):446–54.
Evans SR. Fundamentals of clinical trial design. J Exp Stroke Transl Med. 2010;3(1):19–27.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Furberg BD. Furberg CD. What are the strengths ofrandomized controlled clinical trials? In: Furberg BD, Furberg CD editors. Evaluating clinical research, the second edition. Springer; 2007, pp: 11–16.
Bornhoft G, Maxion-Bergemann S, Wolf U, Kienle GS, Michalsen A, Vollmar HC, et al. Checkist for the qualitative evaluation of clinical studies with particular focus on external validity and model validity. BioMed Central Med Res Methodol. 2006;6:56.
Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244. https://doi.org/10.1136/bmj.b3244.
Fisher L, Hesler D, Naranjo D, Polonsky W. AASAP: a program to increase recruitment and retention in clinical trials. Patient Educ Couns. 2012;86:372–7.
Joseph SW. Problems in dealing with missing data and informative censoring in clinical trials. Curr Control Trials Cardiovasc Med. 2002;3(1):4. https://doi.org/10.1186/1468-6708-3-4.
Sakpal T. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1(2):67–9.
Leathem CS, Cupples ME, Byrne MC, O'Malley M, Houlihan A, Murphy AW, et al. Identifying strategies to maximise recruitment and retention of practices and patients in a multicentre randomised controlled trial of an intervention to optimise secondary prevention for coronary heart disease in primary care. BMC Med Res Methodol. 2009;9:40. https://doi.org/10.1186/1471-2288-9-40.
Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:MR000013. https://doi.org/10.1002/14651858.MR000013.pub6.
McCullagh MC, Sanon MA, Cohen MA. Strategies to enhance participant recruitment and retention in research involving a community-based population. Appl Nurs Res. 2014;27(4):249–53. https://doi.org/10.1016/j.apnr.2014.02.007.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J. 2011;162(5):818–25.e6. https://doi.org/10.1016/j.ahj.2011.08.006.
Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4(2):e003821. https://doi.org/10.1136/bmjopen-2013-003821.
Germini F, Marcucci M, Fedele M, Galli MG, Heath T, Mbuagbaw L, et al. Quality of reporting in abstracts of RCTs published in emergency medicine journals: a systematic survey of the literature suggests we can do better. Emerg Med J. 2019. pii: emermed-2019-208629. https://doi.org/10.1136/emermed-2019-208629.
Rabinowitz J, Levine SZ, Barkai O, Davidov O. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–88. https://doi.org/10.1093/schbul/sbn005.
Furberg BD, Furberg CD. What happened to the study subjects who disappeared from the analysis? In: Furberg BD, Furberg CD, editors. Evaluating clinical research; the second edition. Springer; 2007, pp: 76–81.
Funding
This study has been supported by “Diabetes Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences grant numbered 1396-02-97-2161. All of authors thank the Diabetes Research Center for its financial support.
Author information
Authors and Affiliations
Contributions
SHM did literature bibliography, and drafted some parts of the paper. OTM did literature bibliography, reviewed data, wrote draft and conceived the paper. MP, HSE, MKH, EN, ZN, KKH, MA extracted data, and drafted some parts of the paper. BL conceived, and edited the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
All authors declare that there is no conflict of interests. In addition, all authors declare that the supporting fund has not been influenced on submitted manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 221 kb)
Rights and permissions
About this article
Cite this article
Mohseni, S., Tabatabaei-Malazy, O., Peimani, M. et al. Withdrawal reasons of randomized controlled trials on type 2 diabetes: a systematic review. DARU J Pharm Sci 29, 39–50 (2021). https://doi.org/10.1007/s40199-020-00380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-020-00380-7