Abstract
Introduction
This study aims to summarize the retinal and choroidal microvascular features detected by optical coherence tomography angiography (OCTA) in the affected and fellow eyes of patients with retinal vein occlusion (RVO).
Methods
A comprehensive search of the PubMed, Embase, and Ovid databases was conducted to identify studies comparing OCTA metrics among RVO, RVO-fellow, and control eyes. Outcomes of interest included parameters related to foveal avascular zone (FAZ) and fovea- and optic nerve head (ONH)-centered perfusion measurements of superficial capillary plexus (SCP), deep capillary plexus (DCP), and choriocapillaris layer. Pooled results were presented as mean differences or standardized mean differences with 95% confidence intervals.
Results
Fifty-three studies, comprising 2119 RVO, 1393 fellow, and 1178 control eyes, were included in the quantitative meta-analysis. RVO eyes exhibited larger FAZ areas, increased FAZ acircularity, and reduced macular retinal and choriocapillaris perfusion compared to RVO-fellow and control eyes (P < 0.05). RVO eyes also demonstrated significantly lower perfusion density (PD) in the inside-disk and peripapillary regions of the radial peripapillary capillary layer (RPC), as well as lower retinal and choriocapillaris PD in the 4.5 × 4.5 mm2 field of view (FOV) of ONH-centered scans (P < 0.05). RVO-fellow eyes showed decreased SCP-PD and DCP-PD in the parafoveal region and the 3 × 3 mm2 FOV, reduced inside-disk and 4.5 × 4.5 mm2 FOV RPC-PD (P < 0.05), and a diminished choriocapillaris flow area in the 3 × 3 mm2 FOV (P < 0.05).
Conclusions
Both RVO-affected and RVO-fellow eyes exhibited retinal and choriocapillaris microvascular impairment around the fovea and ONH. OCTA represents a promising tool for comprehensively assessing vascular alterations in RVO and providing evidence of fellow eye involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While optical coherence tomography angiography (OCTA) has been used to assess retinal vein occlusion (RVO), key issues remain unresolved, including limited data on directly comparing uninvolved fellow eyes to healthy controls, sparse choroidal assessments, and inconsistent findings across previous studies utilizing various measurement regions and fields of view. |
In this study, we conducted a systematic review and meta-analysis to summarize the microvascular alterations assessed by OCTA parameters of the retinal and choroidal sublayers in both affected and fellow eyes of patients with RVO, focusing on their regional and vascular layer-specific change patterns. |
What was learned from the study? |
Our findings revealed that RVO eyes showed decreased retinal macular and peripapillary perfusion, along with reduced choriocapillaris perfusion, while RVO-fellow eyes also demonstrated reduced perfusion in the retina and choriocapillaris compared to healthy controls. |
This study provides a comprehensive overview of RVO-related vascular alterations, potentially advancing the understanding of RVO pathogenesis and thus treatment strategies. OCTA is also highlighted as a promising tool for evaluating RVO and emphasizing evidence of fellow eye involvement. |
Introduction
Retinal vein occlusion (RVO) is the second most prevalent retinal vascular disease following diabetic retinopathy, affecting approximately 0.77% of the global population aged 30–89 years [1]. RVO is primarily classified into two types based on the occlusion sites: branch RVO (BRVO) and central RVO (CRVO), with the latter being less common but associated with more severe visual impairment [1]. The pathogenesis of RVO is multifactorial, with Virchow’s triad suggesting three contributing mechanisms: venous compression at artery and vein crossings, vessel wall degeneration, and hypercoagulability [2]. RVO and its complications, such as macular edema—caused by the breakdown of the vessel barrier and subsequent leakage of plasma components into the extracellular space—can result in varying degrees of visual impairment [3]. If left untreated, RVO may eventually progress to severe complications like neovascularization, vitreous hemorrhage, and tractional retinal detachment [4].
Traditionally, fundus fluorescein angiography has been the gold standard for diagnosing RVO [5]. However, its limitations, including invasiveness, the risk of allergic reactions, prolonged imaging duration [6], and limited depth resolution that cannot differentiate capillary plexus layers [7], should not be overlooked. Recently, optical coherence tomography angiography (OCTA) has emerged as a crucial diagnostic tool owing to its distinct advantages over traditional imaging techniques. OCTA offers dye-free, noninvasive, and volumetric visualization of the fundus vasculature by detecting the motion contrast of flowing erythrocytes [8]. It provides micron-scale resolutions that allow for the clear visualization of different vessel layers in the retinal vasculature, from the radial peripapillary capillary (RPC) to the deep capillary plexus (DCP) [9], as well as the choroidal system. Consequently, OCTA has become instrumental in the detection of pathological changes associated with RVO progression [10, 11].
Several unresolved issues persist regarding the evaluation of OCTA metrics in RVO. Firstly, most studies concentrated on the retinal microvasculature of affected RVO eyes [12], using the uninvolved fellow eyes as controls for comparison. This approach raises concerns about whether the systemic vascular abnormalities linked to RVO could influence both eyes, highlighting the necessity for a direct comparison between fellow eyes and healthy controls. Secondly, while prior researchers have pointed to potential changes in macular perfusion density due to RVO [13], there is a notable lack of synthesis across different macular subregions. Thirdly, the examination of peripapillary microvasculature has received insufficient attention, with existing studies presenting conflicting findings on vascular alterations around the optic nerve head (ONH) [14, 15]. Fourthly, the limited sample sizes in previous studies may restrict the generalizability of their conclusions, and their evidence levels may not be robust enough to serve as reliable references. Lastly, the application of OCTA in RVO eyes lacks standardization, leading to a fragmented understanding that encompasses various patient subtypes, measurement regions, and analysis methods. Given these considerations, a comprehensive meta-analysis is warranted to consolidate all related evidence, offering a clearer picture of angiographic changes in patients with RVO.
Consequently, we conducted a systematic review and meta-analysis of comparative studies to provide an in-depth overview of OCTA features in both the affected and fellow eyes of patients with RVO. Identifying discriminative patterns of RVO-related alterations among different regions and vascular layers, as well as recognizing significant changes in fellow eyes, may significantly advance our understanding of RVO pathogenesis.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Since the article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, ethics statements are not applicable.
Method of Literature Search
We conducted extensive literature searches using PubMed, Embase, and Ovid databases to identify pertinent studies published from the inception of the databases to December 5, 2023. The key retrieval terms employed in the search strategy were “retinal vein occlusion” and “OCTA”. Detailed search criteria for PubMed are provided in Supplementary Materials: Table S1.
Inclusion and Exclusion Criteria
Clear inclusion and exclusion criteria were established prior to the study selection. Pre-specified inclusion criteria comprised clinical studies reporting quantitative OCTA outcomes through at least one comparison among the following three groups: RVO, fellow, and control eyes. Studies not meeting these criteria were excluded. Additional exclusion criteria included (1) patients with RVO with other ocular diseases potentially confounding the effects of RVO on OCTA outcomes, such as diabetic retinopathy, hypertensive retinopathy, retinal arterial occlusion, glaucoma, uveitis, etc.; (2) reviews, systematic review or meta-analyses, editorials, case reports, or conference abstracts lacking exhaustive data; (3) case reports or studies with a sample size less than 5; (4) animal studies; (5) redundant publications; (6) articles not available in full-text form; (7) non-English articles except those in Chinese, as we can understand their contents accurately.
Data Collection and Quality Assessment
After removing duplicates using EndNote 21 software (Clarivate Analytics, Philadelphia, USA), two researchers (LXW and QZ) independently screened the remaining articles at the title and abstract levels, and subsequently at the full-text level. Any discrepancies between the two researchers (LXW and QZ) during the study selection process were resolved through discussion and mutual consensus, with a third, more experienced researcher (YXC) consulted when necessary. Data of interest was independently extracted from the selected literature by two researchers (LXW and QZ) following a predefined procedure. Detailed characteristics of included studies were collected, including the first author, publication year, region, study design, sample size of each concerned group, mean duration and type of RVO, OCTA devices, and their scan sizes. Meanwhile, the imaging methodologies of included studies were further illustrated in Supplementary Materials: Table S2.
The methodological quality of studies included in the quantitative meta-analysis was evaluated by the same two independent researchers (LXW and QZ) using the criteria recommended by the Agency for Healthcare Research and Quality (AHRQ) to assess the risk of bias. Any conflicts were resolved through discussion or consultation with the third researcher (YXC).
Outcomes of Interest
OCTA metrics were classified into foveal avascular zone (FAZ)-related parameters, fovea-centered and ONH-centered perfusion measurements of retinal capillary layers and choriocapillaris (CC). FAZ-related parameters comprised the FAZ area, perimeter, acircularity, and perfusion density (PD) within a 300-μm-wide annulus surrounding FAZ (FD-300). FAZ acircularity was defined as the deviation degree of FAZ shape from a perfect circle. Given that the terminology for evaluating perfusion conditions differed across OCTA devices, this meta-analysis standardized the nomenclature. For example, “vessel density” might represent either the ratio of vessel flow area to the total area or the ratio of vessel flow length to the total area. Therefore, we designated the area ratio as “PD” and the latter as “vessel length density (VLD)” [16].
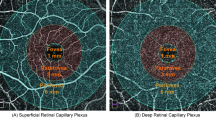
Reported results from the included articles were categorized according to the measured layers and subregions. Retinal capillary layers were divided into the superficial capillary plexus (SCP) and DCP, along with RPC only for ONH-centered scans. Besides the whole FOVs of 3 × 3 mm2, 4.5 × 4.5 mm2, and 6 × 6 mm2, for fovea-centered scans, subregions of “fovea”, “parafovea”, and “perifovea” were also involved, respectively designated as the 1-mm circular region, 1- to 3-mm annular region, and 3- to 6-mm annular areas. The parafoveal annulus was further divided into the superior, inferior, temporal, and nasal quadrants. For ONH-centered scans, the measured subregions included the “inside-disk” and “peripapilla” areas, defined as the 2-mm circular region and the 2- to 4-mm annular region. The peripapillary area could be further subdivided into eight subregions according to the Garway-Heath method [17].
Data Analyses
Review Manager V.5.4 (Cochrane Collaboration, Oxford, UK) was used for data synthesis, presenting mean differences (MD) or standardized mean differences (SMD) along with their 95% confidence intervals (CIs) for continuous variables. The I2 statistic was employed to evaluate statistical heterogeneity. The random-effects model was adopted for meta-analysis if I2 values exceeded 50%; otherwise, the fixed-effects model was used. Sensitivity analysis was performed using Stata 17.0 (StataCorp, College Station, TX, USA) by sequentially excluding one study at a time to assess the stability of the pooled results. Publication bias was evaluated by Egger’s linear regression test. P < 0.05 indicated a significant difference.
Results
Study Characteristics
Our literature search identified 1003 potentially relevant articles. Following duplicate removal and title/abstract screening, 196 full-text articles were reviewed. Among these, 65 studies underwent narrative synthesis, with 53 studies, comprising 4690 eyes, included in the quantitative meta-analysis (see Fig. 1 and Supplementary Materials: Text S1). These studies involved 2119 RVO eyes (1503 BRVO, 511 CRVO, and 105 unspecified), 1393 RVO-fellow eyes, and 1178 healthy control eyes. Detailed characteristics of all included studies are presented in Table 1.
FAZ-Related Parameters
Significantly larger full-retinal FAZ areas and lower FD-300 were found in RVO eyes than RVO-fellow and controls, as well as in CRVO eyes than controls (P < 0.05). BRVO eyes exhibited lower FD-300 than BRVO-fellow eyes and controls, alongside a significantly larger full-retinal FAZ perimeter (P < 0.05). Both RVO and BRVO eyes demonstrated significantly increased full-retinal acircularity when compared to fellow and control eyes (P < 0.05). No significant differences in the FAZ-related parameters were observed between RVO-fellow eyes and controls (P > 0.05, see Fig. 2). The detailed pooled results of FAZ area in SCP and DCP are displayed in Supplementary Materials: Fig. S1.
Group comparisons of FAZ metrics in OCTA measurements. OCTA parameters including FAZ area, perimeter, acircularity and FD-300 are compared among RVO, fellow, control eyes, whose results are displayed by mean difference and 95% CI. The publication bias of each pooled result is shown by Egger’s linear regression test result. The bold font indicates that the result is significant. When RVO eyes are compared to fellow or control eyes, RVO eyes are called “Experiment” and the other two groups are called “Control”; when fellow eyes are compared to control eyes, fellow eyes are called “Experiment” and controls are called “Control”. BRVO retinal vein occlusion, CI confidence interval, CRVO central retinal vein occlusion, DCP deep capillary plexus, FAZ foveal avascular zone, FD-300 PD within a 300-μm-wide annulus surrounding FAZ, OCTA optical coherence tomography angiography, RVO retinal vein occlusion, SCP superficial capillary plexus
Fovea-Centered Perfusion
Foveal SCP-PD was significantly higher in RVO and CRVO eyes than controls, while it was lower in RVO-fellow eyes than in RVO and control eyes (P < 0.05). Significantly lower parafoveal SCP-PD and DCP-PD were found in RVO eyes than in RVO-fellow eyes and controls, as well as in RVO-fellow and CRVO eyes than in controls (P < 0.05). Detailed comparisons in parafoveal subregions recapitulated these findings. BRVO eyes exhibited lower parafoveal SCP-PD than fellow eyes and controls, as well as lower parafoveal DCP-PD than controls (P < 0.05). BRVO eyes also displayed lower perifoveal SCP-PD than fellow eyes (MD − 4.40 [95% CI − 6.65 to − 2.16], P = 0.0001, I2 = 19.0%), while CRVO eyes demonstrated significantly lower perifoveal SCP-PD and DCP-PD than controls (see Figs. 3 and 4).
Group comparisons of SCP-PD in OCTA measurements. Measured subregions for SCP-PD include the fovea, parafovea (further divided as superior, inferior, nasal, and temporal areas), perifovea, 3 × 3 mm2, 4.5 × 4.5 mm2, and 6 × 6 mm2, whose results are displayed by mean difference and 95% CI. The publication bias of each pooled result is shown by Egger’s linear regression test result. The bold font indicates that the result is significant. When RVO eyes are compared to fellow or control eyes, RVO eyes are called “Experiment” and the other two groups are called “Control”; when fellow eyes are compared to control eyes, fellow eyes are called “Experiment” and controls are called “Control”. BRVO retinal vein occlusion, CI confidence interval, CRVO central retinal vein occlusion, OCTA optical coherence tomography angiography, PD perfusion density, RVO retinal vein occlusion, SCP superficial capillary plexus
Group comparisons of DCP-PD in OCTA measurements. Measured subregions for DCP-PD include the fovea, parafovea (further divided as superior, inferior, nasal, and temporal areas), perifovea, 3 × 3 mm2, 4.5 × 4.5 mm2, and 6 × 6 mm2, whose results are displayed by mean difference and 95% CI. The publication bias of each pooled result is shown by Egger’s linear regression test result. The bold font indicates that the result is significant. When RVO eyes are compared to fellow or control eyes, RVO eyes are called “Experiment” and the other two groups are called “Control”; when fellow eyes are compared to control eyes, fellow eyes are called “Experiment” and controls are called “Control”. BRVO retinal vein occlusion, CI confidence interval, CRVO central retinal vein occlusion, DCP deep capillary plexus, OCTA optical coherence tomography angiography, PD perfusion density, RVO retinal vein occlusion
Compared to controls, significantly decreased SCP-PD and DCP-PD were observed in the 3 × 3 mm2 and 6 × 6 mm2 FOVs of RVO, BRVO, and CRVO eyes, as well as in the 3 × 3 mm2 FOV of their fellow eyes (P < 0.05). RVO eyes also exhibited significantly lower SCP-PD and DCP-PD than fellow eyes in 3 × 3 mm2 and 6 × 6 mm2 FOVs (P < 0.05). Significantly decreased SCP-PD and DCP-PD were noticed in BRVO eyes in the 3 × 3 mm2 FOV than in BRVO-fellow eyes, as well as in the 4.5 × 4.5 mm2 FOV than in controls (P < 0.05) (see Figs. 3 and 4). Furthermore, BRVO eyes demonstrated significantly lower SCP-PD and DCP-PD in the affected and unaffected sectors than fellow and control eyes (P < 0.05, see Supplementary Materials: Fig. S2).
Regarding choroidal perfusion in the 3 × 3 mm2 FOV, CC-PD in RVO and CRVO eyes, as well as CC-FA in RVO and RVO-fellow eyes, were significantly lower than in controls (P < 0.05). RVO eyes also exhibited significantly decreased CC-PD relative to RVO-fellow eyes (MD − 4.87 [95% CI − 8.86 to − 0.87], P = 0.02, I2 = 96.0%, see Supplementary Materials: Fig. S3).
Comparisons of vessel length density among groups are shown in Supplementary Materials: Fig. S4.
ONH-Centered Perfusion
Full-retinal peripapillary ONH perfusion significantly decreased in RVO eyes relative to controls (MD − 2.44 [95% CI − 3.53 to − 1.35, P < 0.0001, I2 = 16.0%). Significantly decreased peripapillary and 4.5 × 4.5 mm2 FOV RPC-PD were observed in RVO and BRVO eyes relative to fellow eyes and controls (P < 0.05); the lower peripapillary RPC-PD in BRVO than their fellow eyes mainly existed in the nasal subregion (MD − 2.02 [95% CI − 3.22 to − 0.81, P = 0.001, I2 = 13.0%). Further detailed analyses in Garway-Heath subregions are shown in Supplementary Materials: Fig. S5. Compared to control eyes, BRVO eyes exhibited decreased inside-disk RPC-PD and 4.5 × 4.5 mm2 FOV ONH-PD in SCP, DCP, and CC (P < 0.05). RVO-fellow eyes also displayed significantly lower RPC-PD in the inside-disk region and the 4.5 × 4.5 mm2 FOV than controls (P < 0.05, see Fig. 5).
Group comparisons of ONH-PD in OCTA measurements. Measured layers and subregions for ONH-PD include the peripapilla for the full-retina layer, the inside-disk, peripapilla (further divided as superior, inferior, nasal, and temporal areas), and 4.5 × 4.5 mm2 for the RPC layer, and 4.5 × 4.5 mm2 for the SCP, DCP, and CC layers. The comparison results are displayed by mean difference and 95% CI. The publication bias of each pooled result is shown by Egger’s linear regression test result. The bold font indicates that the result is significant. When RVO eyes are compared to fellow or control eyes, RVO eyes are called “Experiment” and the other two groups are called “Control”; when fellow eyes are compared to control eyes, fellow eyes are called “Experiment” and controls are called “Control”. BRVO retinal vein occlusion, CC choriocapillaris, CI confidence interval, CRVO central retinal vein occlusion, DCP deep capillary plexus, OCTA optical coherence tomography angiography, PD perfusion density, RPC radial peripapillary capillary, RVO retinal vein occlusion, SCP superficial capillary plexus
Additional Analyses
Patients with RVO were also further subdivided according to the presence of macular edema, and the comparison results of OCTA measurements are displayed in Supplementary Materials: Fig. 6. In the sensitivity analysis, most OCTA parameter comparisons remained stable regardless of study exclusion, indicating a robust association. Exceptions are listed in Supplementary Materials: Table S3. Egger’s tests indicated no publication bias in most applicable comparisons. Additionally, the risk of bias assessment of the 53 studies included in the quantitative meta-analysis is summarized in Supplementary Materials: Table S4.
Discussion
This meta-analysis represents, to the best of our knowledge, the first comprehensive quantitative comparison of OCTA parameters among RVO, fellow, and healthy control eyes. Our findings revealed that RVO eyes exhibited larger FAZ areas and reduced macular retinal and choriocapillaris perfusion compared to fellow and control eyes. RVO eyes also demonstrated significantly decreased RPC-PD in the inside-disk and peripapillary regions, as well as lower retinal and choriocapillaris PD in the 4.5 × 4.5 mm2 FOV. RVO-fellow eyes showed decreased SCP- and DCP-PD in the parafoveal region and the 3 × 3 mm2 FOV, reduced inside-disk and 4.5 × 4.5 mm2 FOV RPC-PD, and a diminished choriocapillaris flow area in the 3 × 3 mm2 FOV.
The significantly larger FAZ area detected in BRVO and CRVO eyes than in fellow eyes and controls aligns with previous findings [18]. This enlargement likely arises from pathological changes in vessels surrounding the FAZ, including vascular dilation and tortuosity due to outflow obstruction in RVO. Such alterations can damage terminal segments of the marginal arch ring of the FAZ, reducing perfusion in its vicinity [19], as evidenced by increased FD-300 and FAZ acircularity observed in our study. While FAZ size often indicates foveal circulation status [20], its related parameters exhibit a relatively instable correlation with RVO progression [21].
In contrast, PD offers a more stable reflection of fundus ischemic conditions. Our study revealed significant alterations in both SCP-PD and DCP-PD, with greater reductions often observed in DCP-PD when comparing RVO eyes with fellow or control eyes. This may be due to DCP’s sensitivity to hemodynamic disorders, owing to its direct communication with larger vasculature and lack of vascular smooth muscle cells for autoregulation [22]. Additionally, as the principal venous outflow system for the retinal capillaries, DCP typically develops collateral vessels during RVO evolution [23], negatively associated with retinal PD [24]. Discrepancies in comparison results for the foveal region compared to other regions may arise from different approaches in handling the disruptive effects of FAZ coverage on foveal PD calculation [25]. Regarding PD’s reliability, the increase in blood vessel diameter might mask the capillary dropout or closure in PD measurement, prompting the proposal of VLD to exclude diameter interference with the application of skeletonized images [16]. Despite including several studies reporting VLD, the limited study number made it challenging for us to draw confident conclusions. Thus, more studies are warranted for further investigation into VLD alterations in RVO pathogenesis.
Our findings also support reduced macular CC flow in RVO eyes, just as previously reported [26]. Various mechanisms may underlie the effect of retinal abnormalities on CC flow, including VEGF overproduction [27], choroidal circulation reconstruction [28], and vessel compression due to the outflow of extracellular fluid into the choroid resulting from RVO [29]. However, decreased CC-PD may not solely result from RVO but could be influenced by systemic factors related to microvasculopathies in RVO [12]. CC flow reduction, in return, may disrupt the outer retinal structures nourished by the choroidal circulation, such as the outer limiting membrane and retinal pigment epithelium. Therefore, choroidal involvement in RVO may exacerbate structural damage in affected areas, contribute to RVO progression, and indicate poor visual outcomes.
For monitoring total choroidal tissue, choroidal vascularity index (CVI), the ratio of the luminal vascular component to the total choroid, has been proposed as a valuable metric [30]. In BRVO eyes, OCTA-measured CVI was found to be significantly higher only in specific 1 × 1 mm2 grids of the non-affected hemisphere [28]. This suggests that larger vessels in adjacent areas of occluded regions may dilate to aid blood drainage from affected areas. The hypothesis that large choroidal vessels are less affected by RVO was also supported by Aribas et al. [29], who also observed increased Haller layer to choroidal thickness ratio in RVO eyes, suggesting that high intravascular pressure might protect calibers of the Haller layer from collapse due to choroidal congestion. However, limited relevant reports precluded the meta-analysis for CVI, highlighting the need for further studies to explore alterations in the large and medium choroidal vessel layers in patients with RVO.
In contrast to macular PD, perfusion metrics measured in ONH-centered OCTA images are less susceptible to confounding factors from the macula, such as severe cystoid macular edema. ONH-related parameters not only offer more accurate information amidst serious macular complications but also serve as significant biomarkers for macular retinal perfusion [9]. The RPC in ONH scans represents the most superficial layer of capillaries within the inner part of the retinal nerve fiber layer (RNFL), radiating from the ONH [14]. These capillaries, with long parallel paths and rare anastomoses, are considered particularly vulnerable to abnormal intraocular pressure or hemodynamics, potentially impacted by RVO before noticeable changes occur in other retinal capillary networks [15]. Moreover, RPC nourishes the RNFL surrounding the ONH [31], influencing its structural features and thus aiding in predicting the visual prognosis of RVO. Previous OCTA findings have consistently reported lower RPC-PD in all peripapillary subregions of affected eyes in patients with RVO [14]. However, discrepancies in RPC-PD calculation methods exist across studies, particularly regarding the distinction between capillaries and other large vessels around the optic disc [32, 33]. Our study revealed significantly decreased RPC-PD in RVO eyes than fellow eyes in the peripapillary and 4.5 × 4.5 mm2 regions, but not inside the disk. This may be explained by the presence of vasculature supplying the ONH itself, known as the circle of Haller and Zinn, which partially flows into the central retinal vein and partially into the choroid [9]. This vascular system, considered part of RPC during OCTA analysis, may render RPC-PD less affected by RVO within the inside-disk region [34].
Although lacking sufficient evidence for diagnosing RVO, fellow eyes of patients with unilateral RVO have exhibited preclinical vascular abnormalities in OCTA. Our study identified decreased macular and peripapillary microvascular density in specific regions of fellow eyes, consistent with previous comparisons [35, 36]. Additionally, structural alterations such as thinner peripapillary RNFL thickness in RVO-fellow eyes have been reported [37]. However, Szigeti et al. [38] reported that retinal structural changes may remain undetectable despite the presence of microvascular changes. Fellow eyes of unilateral patients with RVO, theoretically, are at higher risk of evolving into RVO than in healthy controls as a result of systemic risk factors associated with RVO, such as hypertension, hyperlipidemia, and diabetes mellitus [39]. As hypothesized by Zhao et al. [40], RVO might suggest broader systemic vascular alterations affecting both eyes rather than being a solely local disorder. On the one hand, our results on the microvascular alterations in so-called unaffected eyes underscore the importance of regular fundus examination for both eyes of patients with RVO. On the other hand, future studies are warranted to explore whether RVO fellow eyes have distinct characteristics compared to control eyes from patients without RVO but with similar systemic vascular conditions, which might be helpful to discriminate the impact of RVO from systemic disorders on the contralateral vasculopathies. Our findings also revealed that even unaffected sectors of BRVO eyes exhibited significantly decreased PD compared to corresponding areas of fellow or control eyes, albeit to a lesser extent than affected sectors. PD reduction and nonperfusion areas in uninvolved regions have also been reported previously [41]; Lee et al. further hypothesized that reduced branch number and increased tortuosity in large vessels also existed in unaffected vessel branches. These findings supported the hypothesis that vein occlusion in one vessel branch may affect the vasculature of wider regions, underscoring the need for increased clinical attention to the perfusion condition of so-called unaffected areas.
Although the OCTA technique has successfully quantified healthy vasculature and delineated pathologies in a variety of diseases including RVO, understanding the associated artifacts that may skew measurement outcomes or complicate image interpretation in OCTA images is of great significance. Previous studies have noticed that both motion artifacts and segmentation errors are more frequent in eyes with pathology compared to the healthy control eyes, hindering the accurate retinal and choroidal assessment [42, 43]. Besides, the prevalence of different artifact types may also vary significantly across scan sizes even within the same OCTA system [44]. Although many studies included in our meta-analysis have utilized software for the removal of projection artifacts or have implemented exclusion criteria for images with pronounced artifacts, it remains imperative to exercise caution regarding the potential varied impacts of artifacts in each study when interpreting the pooled results. Several efforts have been made to correct the artifacts generated in OCTA images. For example, a self-navigated motion correction technique is under development to assess the impact of motion artifacts on en face OCTA images, enabling automatic and real-time re-scanning of images with excessive motion or blinking artifacts [45]. Furthermore, slab subtraction has been employed to definitively eliminate projection artifacts, thereby reducing the SCP signal’s influence on angiograms in deeper tissues; however, this method may also remove actual flow signals that align with artifact signals, potentially resulting in underestimation of DCP perfusion [46]. Beyond the management of artifact generation, it is also crucial to acknowledge other inherent limitations of OCTA, such as its provision of a cross-sectional view of blood perfusion rather than the comprehensive hemodynamic information.
Our study is not without limitations. First, variability among included eyes, including differences in age, axial length, refractive error [47], and RVO duration [48], may pose challenges when comparing images across different cohorts. Additionally, limited data hindered subgroup analyses to further explore heterogeneity when attempting to compare OCTA parameters among smaller populations meeting specific classification standards. Secondly, the heterogeneity of OCTA devices used in the included articles needs to be taken into account when extending the pooled results. Thirdly, some pooled results showed publication bias in Egger’s test, although the reasons behind this bias were not explored in detail. Finally, a considerable number of pooled results, including those from two studies, did not remain stable in the sensitivity analysis.
Conclusions
This meta-analysis indicated that RVO-affected and RVO-fellow eyes demonstrated retinal and choriocapillaris microvascular impairment around the fovea and ONH. Our findings also revealed that OCTA can represent a promising tool in comprehensively assessing vascular alterations along with RVO progression and providing evidence of fellow eye involvement.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Song P, Xu Y, Zha M, Zhang Y, Rudan I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health. 2019;9(1): 010427.
Marcinkowska A, Cisiecki S, Rozalski M. Platelet and thrombophilia-related risk factors of retinal vein occlusion. J Clin Med. 2021;10(14):3080.
Yin S, Cui Y, Jiao W, Zhao B. Potential prognostic indicators for patients with retinal vein occlusion. Front Med (Lausanne). 2022;9:839082.
Özçalışkan Ş, Özcan Y, Artunay Ö. Macular optical coherence tomography angiography parameters in central retinal vein occlusion and differentiation of ischemic and non-ischemic types. Haseki Tip Bulteni. 2021;59(1):68–73.
Chen L, Yuan M, Sun L, Wang Y, Chen Y. Evaluation of microvascular network with optical coherence tomography angiography (OCTA) in branch retinal vein occlusion (BRVO). BMC Ophthalmol. 2020;20(1):154.
Ghassemi F, Fadakar K, Berijani S, Babeli A, Gholizadeh A, Sabour S. Quantitative assessment of vascular density in diabetic retinopathy subtypes with optical coherence tomography angiography. BMC Ophthalmol. 2021;21(1):82.
Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50.
Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382–9.
Yousif H, Rashad M, Abdel Dayem HK, Abdellatif MK. Evaluation of optic disk and macular vascularity changes in CRVO using optical coherence tomography angiography. Retina. 2023;43(7):1182–8.
Ryu G, Park D, Lim J, van Hemert J, Sagong M. Macular microvascular changes and their correlation with peripheral nonperfusion in branch retinal vein occlusion. Am J Ophthalmol. 2021;225:57–68.
Sakimoto S, Kawasaki R, Nishida K. Retinal neovascularization-simulating retinal capillary reperfusion in branch retinal vein occlusion, imaged by wide-field optical coherence tomography angiography. JAMA Ophthalmol. 2020;138(2):216–8.
Fan L, Zhu Y, Liao R. Evaluation of macular microvasculature and foveal avascular zone in patients with retinal vein occlusion using optical coherence tomography angiography. Int Ophthalmol. 2022;42(1):211–8.
Kim JT, Chun YS, Lee JK, Moon NJ, Yi DY. Comparison of vessel density reduction in the deep and superficial capillary plexuses in branch retinal vein occlusion. Ophthalmologica. 2020;243(1):66–74.
Atilgan CU, Goker YS, Hondur G, Kosekahya P, Kocer AM, Citirik M. Evaluation of the radial peripapillary capillary density in unilateral branch retinal vein occlusion and the unaffected fellow eyes. Ther Adv Ophthalmol. 2022;14:25158414221090092.
Yin S, Cui Y, Jiao W, Zhao B. Quantitative assessment parameters of peripapillary regions with branch retinal vein occlusion by using optical coherence tomography angiography. Biomed Res Int. 2022;2022:9281630.
Zhang B, Chou Y, Zhao X, Yang J, Chen Y. Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol. 2021;222:226–37.
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–15.
Li Z, Gu X, Song S, Yu X, Zhang P, Dai H. Structural and visual changes in branch retinal vein occlusion patients with retinal atrophy. J Ophthalmol. 2022;2022:8945467.
Zhang ZR, Zang DX, Ding XX, et al. Analysis of macular microcirculation and structural features of retinal branch vein occlusion. Int Eye Sci. 2021;21(5):910–4.
Wons J, Pfau M, Wirth MA, Freiberg FJ, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in retinal vein occlusion. Ophthalmologica. 2016;235(4):195–202.
Battaglia Parodi M, Arrigo A, Antropoli A, et al. Deep capillary plexus as biomarker of peripheral capillary nonperfusion in central retinal vein occlusion. Ophthalmol Sci. 2023;3(2):100267.
Coscas F, Glacet-Bernard A, Miere A, et al. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016;161:160–71.
Freund KB, Sarraf D, Leong BCS, Garrity ST, Vupparaboina KK, Dansingani KK. Association of optical coherence tomography angiography of collaterals in retinal vein occlusion with major venous outflow through the deep vascular complex. JAMA Ophthalmol. 2018;136(11):1262–70.
Tsuboi K, Sasajima H, Kamei M. Collateral vessels in branch retinal vein occlusion: anatomic and functional analyses by OCT angiography. Ophthalmol Retina. 2019;3(9):767–76.
Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina. 2017;37(9):1700–9.
Arrigo A, Aragona E, Lattanzio R, Scalia G, Bandello F, Parodi MB. Collateral vessel development in central and branch retinal vein occlusions are associated with worse visual and anatomic outcomes. Invest Ophthalmol Vis Sci. 2021;62(14):1.
Alis A, Guler AM. The effect of branch retinal vein occlusion on the vascular structure of the choroid. Photodiagn Photodyn Ther. 2022;37:102687.
Chen L, Yuan M, Sun L, Chen Y. Three-dimensional analysis of choroidal vessels in the eyes of patients with unilateral BRVO. Front Med (Lausanne). 2022;9:854184.
Aribas YK, Hondur AM, Tezel TH. Choroidal vascularity index and choriocapillary changes in retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2389–97.
Zhao Q, Wang C, Meng L, et al. Central and peripheral changes in the retina and choroid in patients with diabetes mellitus without clinical diabetic retinopathy assessed by ultra-wide-field optical coherence tomography angiography. Front Public Health. 2023;11:1194320.
Antropoli A, Bianco L, Arrigo A, Bandello F, Battaglia PM. Non-perfusion severity correlates with central macular thickness and microvascular impairment in branch retinal vein occlusions. Eur J Ophthalmol. 2024;34(1):226–32.
Shin YI, Nam KY, Lee SE, et al. Changes in peripapillary microvasculature and retinal thickness in the fellow eyes of patients with unilateral retinal vein occlusion: an OCTA study. Invest Ophthalmol Vis Sci. 2019;60(2):823–9.
Wang LY, Liu CQ, Liu JL, et al. Clinical study of retinal vein occlusion papillary area with quantified OCTA. Int Eye Sci. 2020;20(12):2163–6.
Raviselvan M, Preethi B, Ratra D. Retinal perfusion density can predict cardiovascular disease risk in patients with retinal vein occlusion. Indian J Ophthalmol. 2023;71(2):379–84.
Maltsev DS, Kulikov AN, Kazak AA, Burnasheva MA. Status of choriocapillaris in fellow eyes of patients with unilateral retinal vein occlusions. Ophthalmic Surg Lasers Imaging Retina. 2021;52(1):23–8.
Ozcaliskan S, Ozcan Y. Quantitative assessment of macular microvasculature and radial peripapillary capillary plexus in the fellow eyes of patients with retinal vein occlusion using OCT angiography. J Fr Ophtalmol. 2020;43(9):842–50.
Kim MJ, Woo SJ, Park KH, Kim TW. Retinal nerve fiber layer thickness is decreased in the fellow eyes of patients with unilateral retinal vein occlusion. Ophthalmology. 2011;118(4):706–10.
Szigeti A, Schneider M, Ecsedy M, Nagy ZZ, Récsán Z. Optic disc morphology in unilateral branch retinal vein occlusion using spectral domain optical coherence tomography. BMC Ophthalmol. 2015;15:178.
Scott IU, Campochiaro PA, Newman NJ, Biousse V. Retinal vascular occlusions. Lancet. 2020;396(10266):1927–40.
Zhao XY, Zhao Q, Wang CT, et al. Central and peripheral changes in retinal vein occlusion and fellow eyes in ultra-widefield optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2024;65(2):6.
Lee H, Kim MA, Kim HC, Chung H. Characterization of microvascular tortuosity in retinal vein occlusion utilizing optical coherence tomography angiography. Sci Rep. 2020;10(1):17788.
Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2017;101(5):564–8.
Cui Y, Zhu Y, Wang JC, et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl Vis Sci Technol. 2019;8(6):18.
Tsai WS, Thottarath S, Gurudas S, Pearce E, Yamaguchi TCN, Sivaprasad S. A comparison of optical coherence tomography angiography metrics and artifacts on scans of different sizes in diabetic macular ischemia. Am J Ophthalmol. 2024:269:303–14.
Wei X, Hormel TT, Guo Y, Hwang TS, Jia Y. High-resolution wide-field OCT angiography with a self-navigation method to correct microsaccades and blinks. Biomed Opt Express. 2020;11(6):3234–45.
Hormel TT, Huang D, Jia Y. Artifacts and artifact removal in optical coherence tomographic angiography. Quant Imaging Med Surg. 2021;11(3):1120–33.
Jung JJ, Chan X, Lim SY, Lee SS, Rofagha S, Hoang QV. Quadrant asymmetry in optical coherence tomography angiography metrics in ischemic versus non-ischemic central retinal vein occlusion eyes. Transl Vis Sci Technol. 2023;12(3):30.
Park YJ, Kim J, Lee EJ, Park KH. peripapillary microvasculature of the retina and choriocapillaris in uninvolved fellow eyes of unilateral retinal vein occlusion patients. Retina. 2022;42(1):159–67.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, commented on previous versions of the manuscript, and approved the final manuscript. Material preparation, data collection and analysis were performed by Linxin Wei and Qing Zhao. The first draft of the manuscript was written by Linxin Wei. Qing Zhao and Youxin Chen revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Linxin Wei, Qing Zhao, and Youxin Chen declare that they have no competing interests.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wei, L., Zhao, Q. & Chen, Y. Detection of Retinal and Choriocapillaris Microvascular Changes in Retinal Vein Occlusion and Fellow Eyes by Optical Coherence Tomography Angiography: A Systematic Review and Meta-Analysis. Ophthalmol Ther 14, 391–411 (2025). https://doi.org/10.1007/s40123-024-01077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-01077-9