Abstract
Refractory coccydynia is a condition characterized by severe coccygeal pain and poses a challenging management dilemma for clinicians. Advancements in neuromodulation (NM) technology have provided benefits to people experiencing chronic pain that is resistant to standard treatments. This review aims to summarize the spectrum of current NM techniques employed in the treatment of refractory coccydynia along with their effectiveness. A review of studies in the scientific literature from 2012 to 2023 was conducted, revealing a limited number of case reports. Although the available evidence at this time suggests significant pain relief with the utilization of NM techniques, the limited scope and nature of the studies reviewed emphasize the need for large-scale, rigorous, high-level research in this domain in order to establish a comprehensive understanding of the role of NM and its effectiveness in the management of intractable coccydynia.
Similar content being viewed by others
This review highlights the potential effectiveness of neuromodulation as a therapeutic option for patients resistant to conventional treatments. |
Neuromodulation may offer a novel approach to chronic coccydynia management, warranting further high-level research. |
Most case reports demonstrated significant pain reduction through different neuromodulation techniques. |
This narrative review underscores the need for further research to optimize neuromodulation strategies in coccydynia management. |
Introduction
Overview of Coccydynia
Coccydynia or coccygodynia is a debilitating condition that affects many individuals and can cause significant discomfort and decreased quality of life. The coccyx is located at the bottom of the spine and is susceptible to injury or damage, resulting in pain. It can be caused by a variety of traumatic or non-traumatic factors [1]. When there is an injury, inflammation, or irritation in the coccyx region, nociceptors, specialized pain receptors, are activated. These nociceptors send signals to the nearby somatic nerves. The somatic nerves then transmit these nociceptive signals to the central nervous system, where they are processed, leading to the perception of pain. The ganglion impar or Walther ganglion, which is in the retroperitoneal space behind the rectum around the sacrococcygeal joint or directly in front of the coccyx, may also be involved in the transmission of pain signals [2]. The coccygeal plexus plays a crucial role in providing sensory input from the coccyx and comprises the 4th and 5th sacral nerves and the coccygeal nerve. Neuropathic pain is caused by damage or irritation of these nerves. Additionally, pain in the coccygeal area may also stem from pelvic organs, as pain signals travel through the superior hypogastric plexus consisting of sympathetic fibers. Moreover, the pelvic splanchnic nerves, which carry parasympathetic nerves from the second, third, and occasionally fourth sacral segments, can also convey this type of pain [3, 4].
Refractory coccydynia is characterized by persistent pain in the coccyx that does not respond to conservative or non-surgical treatment approaches and can cause significant discomfort and decreased quality of life. Several studies have shown that chronic pain is linked to synaptic plasticity and alterations in the central nervous system (CNS), impacting neural areas that regulate pain. This condition involves structural and functional changes in the corticolimbic brain region [5,6,7]. In some cases, there is a discrepancy between the underlying cause and the severity of pain. Central sensitization, characterized by amplified neural signaling in the CNS, is considered a potential explanation, leading to hypersensitivity [8, 9].
Management of coccyx pain involves a comprehensive approach of pharmacological and non-pharmacological interventions. The choice of treatment depends on the severity of the condition and the patient’s response to previous treatment [10, 11].
Pharmacological treatments often include the use of nonsteroidal anti-inflammatory drugs (NSAIDs) along with topical analgesics. Opioid medications may be prescribed, particularly in cases of severe pain. In patients with pelvic hypertonia or muscle spasms, muscle relaxants and benzodiazepines are also prescribed. Antidepressants and anticonvulsants are effective for neuropathic and CNS hypersensitivity pain. A systematic review/meta-analysis highlights palmitoylethanolamide (PEA) as a well-tolerated and efficacious treatment for chronic neuropathic and inflammatory pain [12,13,14]. Non-pharmacological treatments emphasize conservative approaches including ergonomic adaptations, physical therapy, manipulation, various injections, and alternative therapy.
Physical therapists may employ modalities and techniques to reduce pain, improve mobility of the coccyx and surrounding tissue structures, and educate patients on lifestyle adjustments such as postural education, work ergonomics, and defecation mechanics. In addition, soft tissue mobilization and manual therapy may help to reduce muscle spasms and enhance the flexibility of surrounding tissues [15, 16].
Local injections around the coccyx, trigger point injections, caudal epidural injections, ganglion impar blocks, and radiofrequency ablation (RFA) can also be administered to alleviate coccyx pain. These injections often contain local anesthetics and corticosteroids [17,18,19].
Some other therapies like extracorporeal shock wave therapy (ESWT), acupuncture, prolotherapy, and botulinum toxin injections have shown promise in treating coccyx pain [20,21,22,23].
In refractory cases, partial or complete coccygectomy may be considered as a last resort [24].
Overview of Neuromodulation Techniques
Neuromodulation (NM) is an umbrella term encompassing a spectrum of relatively new and minimally invasive techniques that have shown promising results in managing various chronic pain conditions. However, there is limited data regarding its efficacy in the treatment of coccydynia.
Several NM methods are available to manage chronic coccydynia, these include spinal cord stimulation (SCS), dorsal root ganglion stimulation (DRG-S), peripheral nerve stimulation (PNS), peripheral nerve field stimulation (PNFS), and sacral neuromodulation (SNM).
The traditional approach to SCS involves using 30–60 Hz of electrical stimulation to activate the dorsal columns, resulting in a tingling sensation or paresthesia in the painful areas of the body experienced by a patient and has been found to be effective in managing persistent pain conditions. SCS, rooted in gate control theory, stimulates non-nociceptive fibers in dorsal columns, closing a spinal gate to relieve pain. It triggers inhibitory interneurons, releasing GABA in the spinal dorsal horn [25,26,27]. Recent studies explore cortical and subcortical involvement. Neuroimaging shows cortical changes, suggesting direct effects from dorsal column stimulation or inhibition of nociceptive signals. Functional magnetic resonance imaging (fMRI) studies reveal modulation of brain regions linked to the spinothalamic tract responsible for pain transmission [28,29,30,31].
High-frequency SCS, also known as HF10, is an innovative form of neurostimulation treatment that employs the use of high-frequency stimulation at low amplitudes, ensuring that the stimulation remains below the sensory activation threshold and is paresthesia-free. Empirical observations have determined that the first electrode of one lead is usually placed at the top of the T8 vertebra and the last electrode of the second lead is placed at the bottom of the T11 vertebra with some overlap of the leads at the T9/T10 disc for managing back and leg pain [32]. The mode of operation involves reducing the activity of wide dynamic range (WDR) neurons, and by delivering high-frequency stimulation, it is possible to decrease WDR responsiveness and thereby alter the perception of chronic spinal pain. This method offers safe and long-lasting relief from pain in groups of individuals suffering from chronic pain [33,34,35].
Burst SCS is another technique that utilizes a burst frequency of 40 Hz and a pulse frequency of 500 Hz, by decreasing the amplitude to alleviate pain while minimizing or eliminating paresthesia [36].
DRG-S is a more focused type of NM due to the unique anatomy and physiology of the DRG. The DRG is located inside the neural foramen and the neurons in the DRG provide very specific dermatomal sensory information. Unique to the DRG is the thin surrounding layer of cerebrospinal fluid which prevents the propagation of current to the spinal cord or ventral root, which may explain the mechanism through which DRG-S relieves neuropathic pain [37,38,39].
PNS refers to the application of electrical stimulation to a particular nerve trunk through implanted electrodes placed under the skin to activate the region of affected nerves, cutaneous afferents, or the dermatomal distribution of the nerves instead of targeting the epidural space or a nerve bundle directly and the stimulation is directed towards nerves that converge back to the spinal cord [40].
PNFS also known as subcutaneous field stimulation is an emerging technology that utilizes electrical impulses to activate the region of affected nerves, cutaneous afferents, or the dermatomal distribution of the nerves instead of targeting the epidural space or a nerve bundle directly and the stimulation is directed towards nerves that converge back to the spinal cord. Some studies have shown the effectiveness of this modality in managing chronic pain [41, 42].
One specific form of NM is SNM, a type of nerve stimulation that aims to induce changes in the functioning of sacral nerves and the signals they transmit to the brain. SNM utilizes electrical stimulation to activate the afferent sacral nerve roots through the implanted electrode percutaneously and can be utilized through different percutaneous approaches such as retrograde lumbar, anterograde sacral hiatus, and transforaminal approaches. Each approach has its pros and cons. The retrograde approach is technically complex, posing risks like dural puncture and cerebrospinal fluid leakage. In contrast, the anterograde approach is simpler but can be challenging because of limited subcutaneous tissue, increasing the chance of skin erosion. The transforaminal approach minimizes dura-related risks compared to retrograde access, but it has a drawback of increased potential for lead migration compared to the sacral hiatus route [43,44,45,46,47,48,49,50].
The process of NM consists of two main steps performed in an outpatient setting. Before undergoing a procedure, it is essential to conduct a psychological assessment. This assessment helps to understand how underlying psychosocial risk factors may impact clinical outcomes and aids in managing patient expectations. In the first step, patients undergo a trial stimulation, which allows them to assess the potential alleviation of pain. Trial therapy is more beneficial for predicting long-term success in patient selection compared to relying solely on clinical screening. This preference stems from the lower confidence associated with clinical screening alone, which can be influenced by various factors. The trial involves a minor surgical procedure where leads are inserted using an epidural Tuohy needle and are linked to a temporary external stimulator. If the therapy proves its effectiveness by typically achieving a more than 50% pain reduction, the next step involves the placement of a permanent system. This requires a small incision to implant the leads and to create a subcutaneous pocket in the buttock area. The leads are connected to an implantable pulse generator (IPG) to provide ongoing stimulation and are positioned within the pocket under fluoroscopy. Patients can also manage the therapy using a wireless programmer.
The procedures share similarities across different NM techniques. However, there are a variety of factors such as trial length and approaches, stimulation settings, the number of leads implanted, the placement of leads, and whether the device is rechargeable that distinguish the modalities from one another [42, 51,52,53,54].
The implanted NM system does not restrict the patient’s activities. However, it is crucial to consider that high-frequency diathermy and unipolar electrocoagulation are not recommended. Additionally, during extracorporeal shockwave lithotripsy, the focal point should not be near the neuromodulator or its lead. Ultrasound and radiotherapy in the area where the implanted components are located should also be avoided. NM should not be used during pregnancy [55].
Ensuring MRI safety for implantable devices is complex but crucial. Manufacturers’ guidelines are necessary for safe scans, and adherence to specific conditions is vital to mitigate risks [56, 57].
Like any medical procedure, NM is not without risks. Complications associated with NM include infection, device malfunction, lead migration, and nerve injury. However, the incidence of these complications is relatively low, and most patients tolerate the procedure well [58, 59].
The aim of this review is to summarize the utility of current NM techniques in treating chronic refractory coccydynia and their respective effectiveness based on the available body of knowledge in the scientific literature at this time.
Methods
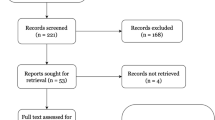
A narrative review of peer-reviewed literature from 2012 to 2023 describing the use of NM techniques to manage coccydynia was conducted. We selected a narrative review methodology because it is the most inclusive review format and thus would permit the most latitude in studying this topic. Medline, Scopus, and Google Scholar databases were used in the literature search. Search terms used in the narrative review are “coccydynia” OR “coccyx pain” OR “coccygodynia” OR “chronic coccydynia” OR “refractory coccydynia” AND “neuromodulation” OR “sacral neuromodulation” OR “spinal cord stimulation” OR “high-frequency spinal cord stimulation” OR “dorsal root ganglion stimulation” OR “peripheral nerve stimulation”. Manual hand searches of relevant journals and publications in addition to posters and abstracts presented at conferences or scientific meetings were also performed. Articles were included if written in English, contained at least one of the keywords in the title, and had a focus on the management of coccydynia.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Twelve publications comprising 13 case reports (level IV evidence) [60,61,62,63,64,65,66,67,68,69,70,71] met the inclusion criteria and have been summarized in Table 1. No level I randomized controlled trials evaluating any of the treatments for coccydynia were located or reviewed.
Five studies showed significant pain reduction in the coccyx and improved quality of life after SNM [61, 62, 68,69,70].
Hope and Gruber [61] used a transforaminal approach through the S3 level and the lead was placed at the left S3 nerve root. Two months after the procedure, the patient lost the remote control and turned off the device which led to a prompt return of her coccygeal pain. However, after accessing a new remote, she rapidly felt better, and the improvement persisted for 3.5 years after implantation.
Auerbach and Lucas [62] utilized the caudal approach through the sacral hiatus to directly stimulate the sacral nerve root at the S1 level on each side. The patient experienced significant pain relief at the 2-month follow-up. The patient reported an 80% reduction in pain and a 40% improvement in functional capacity.
Lee and Lai [68] performed a 5-day trial of percutaneous SCS, utilizing the same pathway through the sacral hiatus for both the trial and implantation. The trial proved to be successful, as the patient experienced significant relief of 80% during its duration. Following the trial, the patient was prescribed BurstDR SCS at a strength of 150 μA.
Waleed Haddad et al. [69] applied a retrograde technique to access the S2–S4 sacral canal (Fig. 1). The patient experienced more than 95% pain relief during the 6-day trial period and as a result the permanent implantable neurostimulator was performed. The patient was comfortable with the SCS setting of frequency 50 Hz, and pulse width range of 300–350 µs.
Lateral view of lead placement (S2–S4 level), from Waleed Haddad et al. [69]
Bawany and Sehgal [70] used a transforaminal approach through S3 (Fig. 2). A trial was accomplished and as a result the patient experienced a 50% improvement in her pain and sleep quality during the 10-day trial. Therefore, she underwent the placement of the permanent peripheral nerve stimulator consisting of four electrodes and wireless receivers. At 12 months post implant, the patient reported 50% relief of symptoms.
Anteroposterior view of lead placement (overlay the distal sacrum and region of the excised coccyx), from Bawany and Sehgal [70]
Four case reports utilized HF SCS; however, the electrodes were placed in different disc spaces spanning T9 to T12 [63, 64, 67].
Simopoulos et al. [67] initiated a trial of 10 kHz SCS. During the trial phase, the patient experienced a 50% decrease in pain levels. Additionally, there was a notable improvement in the patient’s tolerance for sitting (120 min), which had been challenging previously (15 min). Following the permanent procedure, the patient reported an average pain intensity on the visual analog scale (VAS) of 4.0, maintaining a 50% pain reduction.
In the study conducted by Yakovlev and Resch [60], a percutaneous procedure was employed to place two 8-electrode epidural leads. The initial step involved gaining epidural access at the T11/T12 interspace, with the final placement of leads achieved at T8, T9, and T10. During the trial, the patient experienced significant improvement, with pain reduction exceeding 70%. Subsequently, 2 weeks later, the patient underwent the placement of permanent leads along with a rechargeable generator. Following the permanent implantation of leads and the generator, the patient experienced excellent pain relief, leading to the discontinuation of all oral pain medications. Moreover, the patient reported several other positive outcomes, including the ability to resume work, engage in social and sporting activities, and experience improved family relationships.
Giordano et al. [66] utilized bilateral L1 and S2 DRG stimulation (Figs. 3 and 4) that provided significant pain reduction (approximately 100%) during the 7-day trial, prompting permanent lead implantation. The patient reported more than 90% pain relief and improvement in her daily function at 4 months follow-up.
Lateral fluoroscopic image of bilateral dorsal root ganglion leads on the S2 level, from Giordano et al. [66]
Anterior–posterior (AP) fluoroscopic image of dorsal root ganglion leads on the L1 level, from Giordano et al. [66]
Santiago et al. [71] conducted a study utilizing bilateral S3 and S4 DRG lead placements, assisted by tomography-based neuronavigation techniques (Fig. 5). During the 5-day trial, the patient reported significant pain relief upon stimulation initiation. The stimulation parameters were tonic in nature, with a frequency of 20 Hz, a pulse width of 300 μs, and amplitude levels ranging from 0.475 to 1.25. The patient experienced significant pain reduction and noted an overall improvement in their quality of life. They reported a better social life and a successful return to work post stimulation.
Lateral fluoroscopic image of dorsal root ganglion leads on bilateral S3 and S4, from Santiago et al. [71]
Granville et al. [65] inserted two permanent leads within the deep posterior sacral fascia on both sides of the lower sacrum (Fig. 6). The leads were adjusted to establish communication and cross talk from right to left to enhance midline and coccygeal stimulation, leading to effective coccygeal pain relief.
Anterior–posterior intraoperative film of two paramedian sacral peripheral field electrodes. Black arrows indicate the path of each electrode from bottom to top. Two solid arrows depict electrodes tunneled to the generator. Coccyx cement is noted at Cx. Coccygeal cement (open white arrows) is below the entry point of deep fascial electrodes, offering field stimulation in the paramedian sacral area. The dotted black arrow shows sacral alae cement, and sacroplasty cement is indicated by a dashed white arrow. From Granville et al. [65]
Limitations
In the current review, some studies are derived from past meetings and posters. This limits the availability of additional details or a more in-depth discussion regarding the underlying causes, duration of pain, and prior interventions. The lack of high-level evidence including well-designed randomized controlled trials in support of current treatment methods led the authors to present this literature review in the form of a narrative review. Additionally, limited conclusions can be drawn from low-level evidence such as case reports that were included in this review.
Conclusion
Overall, all the study participants with refractory coccydynia underwent a successful trial followed by successful clinical outcomes through a variety of neuromodulation techniques. Given these results, this suggests that neuromodulation may be a viable treatment option for carefully selected candidates with chronic coccydynia that is resistant to conservative therapy and/or surgery. However, given the limited number and scope of studies reported in the scientific literature at this time, more rigorous larger-scale studies need to be conducted in order to assess whether the reduction in pain is predictably reproducible, significant, and sustained for this patient population.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Patel R, Appannagari A, Whang PG. Coccydynia. Curr Rev Musculoskelet Med. 2008;1:223–6.
Gunduz OH, Sencan S, Kenis-Coskun O. Pain relief due to transsacrococcygeal ganglion impar block in chronic coccygodynia: a pilot study. Pain Med. 2015;16(7):1278–81.
Woon JT, Stringer MD. Redefining the coccygeal plexus. Clin Anat. 2014;27(2):254–60.
Woon JT, Stringer MD. Clinical anatomy of the coccyx: a systematic review. Clin Anat. 2012;25(2):158–67.
Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci. 2019;20(13):3130.
Barroso J, Branco P, Apkarian AV. Brain mechanisms of chronic pain: critical role of translational approach. Transl Res. 2021;238:76–89.
Apkarian AV, Reckziegel D. Peripheral and central viewpoints of chronic pain, and translational implications. Neurosci Lett. 2019;29(702):3–5.
Nijs J, George SZ, Clauw DJ, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–92.
Nijs J, Malfliet A, Nishigami T. Nociplastic pain and central sensitization in patients with chronic pain conditions: a terminology update for clinicians. Braz J Phys Ther. 2023;27(3): 100518.
White WD, Avery M, Jonely H, Mansfield JT, Sayal PK, Desai MJ. The interdisciplinary management of coccydynia: a narrative review. PM&R. 2022;14(9):1143–54.
Kerr EE, Benson D, Schrot RJ. Coccygectomy for chronic refractory coccygodynia: clinical case series and literature review. J Neurosurg Spine. 2011;14(5):654–63.
Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381–400 (vi-vii).
Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010;23(2):99–108.
Lang-Illievich K, Klivinyi C, Lasser C, Brenna CTA, Szilagyi IS, Bornemann-Cimenti H. Palmitoylethanolamide in the treatment of chronic pain: a systematic review and meta-analysis of double-blind randomized controlled trials. Nutrients. 2023;15(6):1350.
Emerson SS, Speece AJ 3rd. Manipulation of the coccyx with anesthesia for the management of coccydynia. J Am Osteopath Assoc. 2012;112(12):805–7.
Mohanty PP, Pattnaik M. Effect of stretching of piriformis and iliopsoas in coccydynia. J Bodyw Mov Ther. 2017;21(3):743–6.
Mitra R, Cheung L, Perry P. Efficacy of fluoroscopically guided steroid injections in the management of coccydynia. Pain Physician. 2007;10(6):775–8.
Gonnade N, Mehta N, Khera PS, Kumar D, Rajagopal R, Sharma PK. Ganglion impar block in patients with chronic coccydynia. Indian J Radiol Imaging. 2017;27(3):324–8.
Chen Y, Huang-Lionnet JHY, Cohen SP. Radiofrequency ablation in coccydynia: a case series and comprehensive, evidence-based review. Pain Med. 2017;18(6):1111–30.
Lin SF, Chen YJ, Tu HP, et al. The effects of extracorporeal shock wave therapy in patients with coccydynia: a randomized controlled trial. PLoS One. 2015;10(11):e0142475.
Khan SA, Kumar A, Varshney MK, Trikha V, Yadav CS. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg (Hong Kong). 2008;16(1):27–9.
Halder GE, Scott L, Wyman A, et al. Botox combined with myofascial release physical therapy as a treatment for myofascial pelvic pain. Investig Clin Urol. 2017;58(2):134–9.
Ahadi T, Motaghi M, Sajadi S, et al. Acupuncture in adjunction to corticosteroid injection in coccydynia treatment: a randomized clinical trial. J Complementary Med Res. 2021;11(5):166–72.
Kleimeyer JP, Wood KB, Lønne G, et al. Surgery for refractory coccygodynia: operative versus nonoperative treatment. Spine (Phila Pa 1976). 2017;42(16):1214–9.
Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017;20(6):525–33.
Campbell TS, Johnson JA, Zernicke KA. Gate control theory of pain. In: Gellman MD, editor. Encyclopedia of behavioral medicine. Cham: Springer; 2020. https://doi.org/10.1007/978-3-030-39903-0_1134.
Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18(8):1048–67.
Meuwissen KPV, van der Toorn A, Gu JW, Zhang TC, Dijkhuizen RM, Joosten EAJ. Active recharge burst and tonic spinal cord stimulation engage different supraspinal mechanisms: a functional magnetic resonance imaging study in peripherally injured chronic neuropathic rats. Pain Pract. 2020;20(5):510–21.
Meuwissen KPV, de Vries LE, Gu JW, Zhang TC, Joosten EAJ. Burst and tonic spinal cord stimulation both activate spinal GABAergic mechanisms to attenuate pain in a rat model of chronic neuropathic pain. Pain Pract. 2020;20(1):75–87.
Joosten EA, Franken G. Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. 2020;161 Suppl 1(1):S104–13.
Goudman L, De Groote S, Linderoth B, et al. Exploration of the supraspinal hypotheses about spinal cord stimulation and dorsal root ganglion stimulation: a systematic review. J Clin Med. 2021;10(13):2766.
Russo M, Van Buyten JP. 10-kHz High-frequency SCS therapy: a clinical summary. Pain Med. 2015;16(5):934–42.
Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6-month Australian clinical experience. Pain Physician. 2016;19(4):267.
Chakravarthy K, Richter H, Christo PJ, Williams K, Guan Y. Spinal cord stimulation for treating chronic pain: reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation. 2018;21(1):10–8.
Baranidharan G, Edgar D, Bretherton B, et al. Efficacy and safety of 10 kHz spinal cord stimulation for the treatment of chronic pain: a systematic review and narrative synthesis of real-world retrospective studies. Biomedicines. 2021;9(2):180.
De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 2010;66(5):986–90.
Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuro-function. Neuromodulation. 2013;16(4):304–11.
Abd-Elsayed A, Gilligan C. Chronic pelvic pain and role of dorsal root ganglion stimulation. Pain Pract. 2023. https://doi.org/10.1111/papr.
Ghorayeb JH, Chitneni A, Rupp A, Parkash A, Abd-Elsayed A. Dorsal root ganglion stimulation for the treatment of chronic pelvic pain: a systematic review. Pain Pract. 2023;23(7):838–46.
Lin T, Gargya A, Singh H, Sivanesan E, Gulati A. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020;21(1):S6-12.
Verrills P, Vivian D, Mitchell B, Barnard A. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med. 2011;12(9):1395–405.
Petersen EA, Slavin KV. Peripheral nerve/field stimulation for chronic pain. Neurosurg Clin. 2014;25(4):789–97.
Mahran A, Baaklini G, Hassani D, et al. Sacral neuromodulation treating chronic pelvic pain: a meta-analysis and systematic review of the literature. Int Urogynecol J. 2019;30:1023–35.
Paszkiewicz EJ, Siegel SW, Kirkpatrick C, Hinkel B, Keeisha J, Kirkemo A. Sacral nerve stimulation in patients with chronic, intractable pelvic pain. Urology. 2001;57(6):124.
Abd-Elsayed A, Lee S, King C. Retrograde placement of spinal cord stimulator leads for treating resistant pelvic pain. Saudi J Anaesth. 2017;11(3):366–7.
Greig J, Mak Q, Furrer MA, Sahai A, Raison N. Sacral neuromodulation in the management of chronic pelvic pain: a systematic review and meta-analysis. Neurourol Urodyn. 2023;42(4):822–36.
Richter EO, Abramova MV, Aló KM. Percutaneous cephalocaudal implantation of epidural stimulation electrodes over sacral nerve roots—a technical note on the importance of the lateral approach. Neuromodulation. 2011;14(1):62–7.
Falco FJ, Rubbani M, Heinbaugh J. Anterograde sacral nerve root stimulation (ASNRS) via the sacral hiatus: benefits, limitations, and percutaneous implantation technique. Neuromodulation. 2003;6(4):219–24.
Matzel KE, Chartier-Kastler E, Knowles CH, et al. Sacral neuromodulation: standardized electrode placement technique. Neuromodulation. 2017;20(8):816–24.
Hunter CW, Stovall B, Chen G, Carlson J, Levy R. Anatomy, pathophysiology and interventional therapies for chronic pelvic pain: a review. Pain Physician. 2018;21(2):147–67.
Davanzo J, Brandmeir NJ. Surgical technique and patient selection for spinal cord stimulation for chronic pain. Neurol India. 2020;68(Supplement):S213–7.
Garcia K, Wray JK, Kumar S. Spinal cord stimulation. Treasure Island, FL: StatPearls; 2023. PMID 31985947.
McKenzie-Brown AM. Spinal cord stimulation: placement and management. UpToDate. 2020;26:2020.
Shanthanna H, Eldabe S, Provenzano DA, et al. Evidence-based consensus guidelines on patient selection and trial stimulation for spinal cord stimulation therapy for chronic non-cancer pain. Reg Anesth Pain Med. 2023;48(6):273–87.
Wöllner J, Hampel C, Kessler TM. Surgery illustrated–surgical atlas sacral neuromodulation. BJU Int. 2012;110(1):146–59.
Huang X, Jiang GJ. Magnetic resonance imaging interactions with a sacral neuromodulation system. Neurourol Urodyn. 2021;40(8):1862–8.
Jotwani R, Abd-Elsayed A, Villegas K, et al. Failure of SCS MR-conditional modes due to high impedance: a review of literature and case series. Pain Ther. 2021;10(1):729–37.
Kessler TM, Burkhard FC, Madersbacher H, Kofler A, Poewe W, Kiss G. Safety of prolonged sacral neuromodulation tined lead testing. Curr Med Res Opin. 2008;24(2):343–7.
Force L, da Silva G. Management of complications of sacral neuromodulation. Seminars Colon Rectal Surg. 2017;28(4):173–6.
Yakovlev A, Resch BE. Spinal cord stimulation for the treatment of coccydynia following traumatic closed fracture of sacrum and coccyx and rectal tear. Pain Pract. 2012;1(12):182.
Hope ER, Gruber DD. Coccygeal fracture pain cured by sacral neuromodulation: a case report. Neuromodulation. 2013;16(6):614–7.
Auerbach M, Lucas S. Coccydynia treated with spinal cord stimulation: a case report. American Academy of Pain Medicine Annual Meeting. Pain Med. 2015;16(3):559.
Shaikh T, Desai P. High frequency spinal cord stimulation at 10,000 Hz for back, hip and coccyx pain: a case report. Neuromodulation. 2015;18(6):e107–399.
Vajramani G, Hazelgrove J, Cumming M, Berry N. “High frequency 10 kHz spinal cord stimulation (HF10 SCS) for coccydynia: report of two cases. 20th Annual Meeting of the North American Neuromodulation Society. Neuromodulation. 2017;20(7):e122–335.
Granville M, Brennan PT, Jacobson RE. Bilateral peripheral nerve field stimulation for intractable coccygeal pain: a case study using dual lead intercommunication. Cureus. 2017;9(11):e1832.
Giordano NL, van Helmond N, Chapman KB. Coccydynia treated with dorsal root ganglion stimulation. Case Rep Anesthesiol. 2018;2018(1):1–4.
Simopoulos T, Yong RJ, Gill JS. Treatment of chronic refractory neuropathic pelvic pain with high-frequency 10-kilohertz spinal cord stimulation. Pain Pract. 2018;18(6):805–9.
Lee DW, Lai A. Sacral burst neuromodulation via caudal approach as a treatment for chronic coccydynia. Neuromodulation. 2019;22(8):v992–4.
Waleed Haddad H, Elkersh MA, Hankey PB, Kaye AD. Spinal cords stimulation of the sacral region as a treatment for intractable coccygodynia: a case study. Pain Med Case Rep. 2021;5(6):319–23.
Bawany MH, Sehgal N. Successful treatment of chronic coccydynia with peripheral nerve stimulation: a case report. Pain Med Case Reports. 2022;6(2):75–80.
Santiago N, Monaco BA, Santos Piedade G, Jagid J, Cordeiro JG. Navigated dorsal root ganglion stimulation (DRGS) for the treatment of chronic refractory coccygodynia: a case report. Cureus. 2023;15(7):e41663.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Sarvenaz Rahimibarghani conceptualized and designed the manuscript, conducted literature searches, and drafted the manuscript. Richard Morgan contributed to literature searches and manuscript drafting, while Jose Juan Diaz supervised and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Sarvenaz Rahimibarghani, Richard Morgan, and Jose Juan Diaz have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rahimibarghani, S., Morgan, R. & Diaz, J.J. Neuromodulation Techniques in Chronic Refractory Coccydynia: A Narrative Review. Pain Ther 13, 53–67 (2024). https://doi.org/10.1007/s40122-023-00572-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00572-4