Abstract
Objectives/Introduction
Superior cluneal neuralgia (SCN) is a distinct cause of lower back and/or leg pain related to pathology of the superior cluneal nerve (SCn). SCN has been termed pseudo-sciatica and is an overlooked differential diagnosis when patients are otherwise presenting with low back and/or radicular pain. Radiofrequency ablation (RFA) is commonly used for denervation of the medial branches of the dorsal root for facet joint syndrome for sacroiliac joint; however, RFA has not been described to ablate the SCn for SCN. Herein, we present a novel interventional minimally invasive approach using RFA of the SCn for SCN in a series of 46 patients.
Methods
Institutional review board approved retrospective chart review was used to collect data for all SCn RFA cases from January 1, 2018, to February 8, 2021. Fluoroscopically guided SCn ablations were performed for patients with a positive “iliac crest point sign,” reproductive of their back and leg pain during physical examination. Sensory stimulation was utilized to confirm RF cannula-probe placement adjacent to the SCn, and motor testing was used to confirm no distal motor response prior to monopolar RF ablation with a Halyard RF Generator (100 mm curved 22G 10 mm active tip RF cannulae). Charts were reviewed for time of analgesia follow-up, duration and degree of analgesia, improvements in patients’ functional capacity, and changes in medication.
Results
Data were reviewed for 51 patients who underwent Scn RFA, 5 of which were lost to follow-up. The remaining 46 patients consisted of 29 women and 17 men with a mean age of 59.4 years; 78.3% (n = 36) had ongoing relief at a mean of 92.1 days follow-up, ranging from 13 to 308 days, with a mean of 92.3% analgesia (SD 15.0%). At a mean of 111.2 days of follow-up, ranging from 42–201 days, 21.7% (n = 10) of patients reported that their pain had returned and had 95% analgesia during that time period (SD 6.7%); 41.3% (n = 19) reported improved activity/gait, 17.4% reported improved mood (n = 8), and 8.7% reported decreased medication use (n = 4). Five patients had minor complications including bruising (1), 2–3 days of soreness on site (2), myofascial pain (1), and quadratus lumborus muscle spasm relieved with trigger point injection (1).
Conclusions
This is the first report of both technique and outcomes for radiofrequency ablation of superior cluneal neuralgia. This series suggests that RFA of the SCn is a suitable intervention for the treatment of SCN; 21.7% of patients reported a mean of 95% analgesia for a mean duration of 111.2 days, and the remaining 78.3% of patients reported ongoing relief with a mean of 92.3% analgesia at last follow-up (mean 92.1 days). There were no serious adverse events.
Similar content being viewed by others
We described a novel technique on RFA of the superior cluneal nerve |
This is an important procedure for treating low back pain |
Overall, all patients reported improvement after procedure with 100% of patients reporting significant analgesia |
Mean analgesia was reported as 92.3–95% analgesia for a mean duration of 92.1–111.2 days |
Introduction
Low back pain with or without leg pain is a common reason for seeking medical care and is the most common cause for limited activity in patients in the US [1]. Although there are many etiologies for low back pain [2,3,4], an often overlooked etiology is superior cluneal neuralgia (SCN) [5,6,7].
SCN was first described in 1957 as a distinct cause of low back pain with groin and/or leg symptoms [8]. Superior cluneal neuralgia (SCN) is a distinct cause of lower back and/or leg pain related to pathology of the superior cluneal nerve (SCn). SCN has been termed pseudo-sciatica and is an overlooked differential diagnosis when patients are otherwise presenting with low back and/or radicular pain.
The superior cluneal nerve (SCn) and its branches have since been described anatomically, with its own medial, intermediate, and lateral branches perforating the thoracolumbar fascia 5–20 mm superiorly from the iliac crest, with the medial branch often identified passing through a rigid osseoaponeurotic orifice formed at the insertion of the thoracolumbar fascia about 7–8 cm from midline at the posterolateral border of the quadratus lumborum muscle [9,10,11,12,13]. Interestingly, the lateral cutaneous branches of the dorsal rami from T12 to L5 were found to variably coalesce to form the branches of the SCn [9, 14,15,16].
Clinically, physical examination for SCN has also been described as compression of a trigger point atop the iliac crest (also reported as the “iliac crest point”) approximately 7–8 cm from midline, with reproduction of the patient’s pain and/or paresthesia, referred to as the “iliac crest point sign," originally described by Maigne, and modified to be performed in a sitting position with some lumbar extension, as the authors found this extension maneuver to more provocative [7, 9, 12, 17]. As noted by Maigne, a trigger point at the thoracolumbar junction could also be found to reproduce the same SCN distribution pain below the iliac crest in some cases [17].
Reported etiologies for SCN include complications of bone graft harvest from the posterior iliac crest for spinal fusion [18,19,20], dynamic myofascial entrapment [21], and idiopathic, potentially degenerative musculoskeletal processes [6, 7].
Diagnostically, local anesthetic nerve blockade has been utilized in an effort to identify SCN or rule it out [5, 22], but the nerve branches themselves are small [9, 10, 14] and distributed variably [9,10,11, 17], with a significant likelihood of false-negative block if an adequately small volume for specificity of anesthetic is applied and a significant likelihood of false-positive block if a large volume of anesthetic is applied, with likely spread to adjacent structures [23].
Hence, microsurgical approaches recommend the utilization of an awake and responsive patient with the use of intra-procedural nerve stimulation to identify the SCn branch(es) responsible for reproduction of the patient’s symptoms [12]. This approach is thought to be more favorable to the classic surgical approach [5, 7, 21, 23], not only because of its potential for improved intraoperative accuracy, but also because of excellent outcomes [12].
Radiofrequency ablation (RFA) is a common interventional pain management neuroablative technique utilized to treat low back pain of various etiologies [24, 25]. Akin to intraoperative nerve stimulation, prior to lesioning any nerve branch, nerve stimulation is performed intra-procedurally with an awake and responsive patient to identify the branches responsible for transmission of painful stimuli [26]. RFA is often used for denervation of the medial branches of the dorsal root for facet joint syndrome [27] or intervertebral discs [28] for sacroiliac joint [24]. Although described for the medial cluneal nerve [29], RFA of the SCn for SCN has not been described to date. Hence, herein we provide a technical procedural report on RFA of the SCn with medium-term clinical outcomes.
Methods
Institutional review board approved retrospective chart review was used to collect data for all SCn RFA cases from January 1, 2018, to February 8, 2021, from Veritas IRB (protocol ID: 2021-2584-5272-1) an IRB accredited by the Human Research Accreditation Canada. All patients were treated by a fellowship-trained board-certified interventional pain management physician. Patients presenting with low back pain received the routine history and physical examination with review of radiologic findings to identify all causes of low back pain.
The diagnosis of SCN was clinically obtained, as described above [7, 9, 12, 17], reproducing the patient’s chief complaint with a positive “iliac crest point sign," originally described by Maigne, and modified to be performed in a sitting position with some lumbar extension, as the authors found this extension maneuver more provocative. In the authors’ experience, this trigger point, or “iliac crest point,” is more easily identified if the patient attempts mild lumbar extension with accentuation of lumbar lordosis while sitting or standing upright prior to application of pressure atop the iliac crest. Those considered to have SCN were then consented for planned RFA and scheduled accordingly. Diagnostic blocks were not performed for the reasons noted above in favor of intra-procedural neurosensory stimulation, akin to that noted above [12].
The overall approach utilized for RFA of the SCn was similar to ablation of medial branches of the dorsal rami or lateral sacral branches [24, 27]. A Halyard Health RF Pain Management Generator (PMG-Advanced, version 4) was utilized. Halyard Health 22-gauge 10-cm RF cannulas with 10-mm active curved tips were used for all procedures. All RF lesions were monopolar.
On the procedure day, each patient was identified, screened, re-assessed to confirm diagnosis and planned procedure, and then positioned prone on the fluoroscopy table with a pillow under the low abdomen to reduce lumbar lordosis. This positioning allowed for improved patient comfort. Each patient was then prepped with three rounds of chlorhexidine 2% in 70% isopropyl alcohol solution with sterile draping to follow. Sterile technique applied to all procedures.
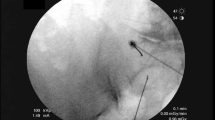
The patient’s skin and subcutaneous tissues were superficially anesthetized with 1–2 ml 2% lidocaine. Using anteroposterior fluoroscopic views (Fig. 1A), in some cases supplemented with oblique views in line with the iliac crest at the site of RF cannula placement, or lateral views, when needle tip placement was questionable and/or sensory capture were elusive, a cannula was advanced to the superior border of the iliac crest targeting the osseoaponeurotic orifice, which appears as a small ossified cephalad protrusion at the posterior iliac crest (Fig. 1C, D, black outline). After osseous contact, the RF cannula was walked off the superior border of the iliac crest 3–5 mm (in an effort to avoid deeper and unwanted penetration of non-target tissues, such as viscera), often superomedial to the osseoaponeurotic orifice, to target the SCn proximal to the portion that traverses the canal. This correlated with the greatest point of tenderness approximately 7–8 cm from midline in most patients. No sedation was administered, and each patient was able to provide feedback throughout the procedure.
Fluoroscopic images of a radiofrequency cannula targeting the superior cluneal nerve. A RF cannula placement cephalad to the osseoaponeurotic orifice. B RF cannula placement medial to the osseoaponeurotic orifice, repositioned after failure to capture concordant paresthesia with sensory stimulation. C Same image as B, with a black circle highlighting the location of the osseoaponeurotic orifice. D Zoomed-in image of the osseoaponeurotic orifice with a black circle highlighting its location
Sensory testing was performed at 50 Hz to < 0.5 V and considered positive if the induced sensation was concordant with the patient’s primary pain complaint. If sensory testing was negative for reproduction of paresthesia concordant with the patient’s pain, the cannula tip was repositioned and sensory testing was repeated (Fig. 1B). Following positive sensory testing, motor testing was performed at 2 Hz at a voltage up to 2 V and considered appropriate if there was no distal motor response. Subsequently, 1–2 ml of 2% lidocaine was administered prior to RF lesioning.
RF lesioning was performed at 80 °C for 90 s. This was followed by an examination to determine whether pain could be elicited through the trigger point noted above (the iliac crest point). If the patient perceived complete analgesia, the needle was removed and a sterile dressing was applied.
If some pain persisted, however, the needle was repositioned a few millimeters medially or superolaterally along the iliac crest to identify adjacent SCn branches [10, 11] contributing to pain generation and/or transmission. Following satisfactory repositioning, the above sensory and motor stimulation following by RFA lesioning was performed as indicated. Again, this was followed by an examination to determine whether pain could be elicited through the trigger point noted above (the iliac crest point). The needle was removed and a sterile dressing applied.
Of note, needle repositioning was typically lateral as it was presumed that the initial branch of the SCn ablated was the medial one but, since fluoroscopy is not a technology that allows for visualizing each of the branches and neurosensory stimulation prior to RF lesioning helps to identify a contributory branch, but does not identify which branch (medial, intermediate, or lateral), in some cases, medial repositioning of the cannula resulted in stimulation that reproduced the residual symptoms.
The patients were scheduled for follow-up and any additional procedures, as indicated. The primary outcome assessed at follow-up was duration and degree of analgesia, reported as a percentage of analgesia by patients at follow-up. Secondary outcomes included self-reported improvements in quality of life, physical or emotional function, and post-procedural complications.
Results
Patient Demographics
Medical records were reviewed for 51 patients who underwent SCn RFA, of which 5 were lost to follow-up. The remaining 46 patients, 29 women and 17 men, with a mean age of 59.4 (range: 31–94) years, were reviewed (Table 1).
Primary Outcome
Among the 46 patients treated (Table 1), 78.3% (n = 36) had ongoing relief at last follow-up (mean 92.1 days, range: 13–308 days), with a mean of 92.3% analgesia (SD 15.0%, p < 0.001) during that period. The remaining 21.7% (n = 10) of patients reported that their pain had returned at time of follow-up, indicating a mean of 95% analgesia (SD 6.7%, p < 0.001) at a mean of 111.2 days of follow-up (range: 42–201 days). One hundred percent of patients reported significant analgesia.
Secondary Outcomes and Complications
In addition to pain relief, 41.3% (n = 19) of patients reported improved activity/gait, 17.4% reported improved mood (n = 8), and 8.7% reported decreased medication use (n = 4). In many cases, in addition to analgesia (Table 1), patients experienced an increase in lumbar mobility, most notably, and improved tolerance of flexion (able to tie their shoes or put on their boots).
Only 10.9% (n = 5) procedures resulted in minor complications including bruising (n = 1), 2–3 days of soreness at site (n = 2), myofascial pain (n = 1), and quadratus lumborum muscle spasm relieved with trigger point injection (n = 1). No serious adverse events (any permanent or lasting injury of any kind) were observed or reported (Table 1).
Discussion
RFA is a common interventional pain management neuroablative technique utilized to treat low back pain of various etiologies [24,25,26,27]. Herein, a novel technique for RFA of the SCn to treat SCN has been described with sensory stimulation to affirm nerve branch capture and concordance of pain, affirmed by each patient. Outcomes presented are quite positive with near-complete analgesia with associated improvements in gait, activity, mood, and analgesic medication use among 46 consecutive patients with medium-term clinical outcome follow-up.
It is notable that diagnosis for inclusion was through clinical assessment rather than by diagnostic block, and intraprocedural sensory stimulation was used to supplement fluoroscopy to localize targeted nerve branches. This resulted in excellent outcomes calling into question the practice of obtaining initial diagnostic blocks.
Furthermore, there were no serious adverse events reported in the 46 patients, suggesting that this approach is quite safe. A total of five (10.9%) patients had minor complications: two had transient site soreness, one had myofascial pain, and one had quadratus lumborum muscle spasm relieved with trigger point injection.
Although these outcomes data are promising for future application of this technique, this initial retrospective analysis has the usual imitations of a retrospective review, which should be recognized. As with other retrospective chart reviews, there was a lack of pre-determined data points for collection, non-congruent times for data point acquisition with variable follow-up periods, and a lack of a randomized controlled comparative arm. Additional prospective analyses will need to be performed to corroborate, optimize, and expand upon these data.
Conclusions
SCN is an often overlooked cause of low back pain with or without leg symptoms. Significant anatomic investigation over the past few decades has elucidated the neuroanatomy of the SCn. While past approaches to management were limited to nerve block or surgical decompression, herein we present a novel, safe, and minimally invasive technique for treatment of SCN with RFA.
Approximately 78.3% of cases had ongoing pain relief at last follow-up (mean 92.1 days post-RFA procedure) with a mean of 92.3% analgesia during that period, while the remaining 21.7% of patients reported 95% mean analgesia for a mean of 111.2 days until their pain returned; 10.9% of cases had minor complications. There were no serious adverse events. Future studies should seek long-term outcomes data with more robust data sets, investigate optimal cannula size and lesioning parameters, and consider differences in outcomes relating to identifiable etiologies.
References
Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press (US), 2011. https://doi.org/10.17226/13172.
Vining RD, Shannon ZK, Minkalis AL, Twist EJ. Current evidence for diagnosis of common conditions causing low back pain: systematic review and standardized terminology recommendations. J Manip Physiol Ther. 2019. https://doi.org/10.1016/j.jmpt.2019.08.002.
Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, van Tulder M, Koes BW. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–803.
Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–47.
Berthelot JM, Delecrin J, Maugars Y, Caillon F, Prost A. A potentially underrecognized and treatable cause of chronic back pain: entrapment neuropathy of the cluneal nerves. J Rheumatol. 1996;23(12):2179–81.
Isu T, Kim K, Morimoto D, Iwamoto N. Superior and middle cluneal nerve entrapment as a cause of low back pain. Neurospine. 2018;15(1):25–32.
Kuniya H, Aota Y, Kawai T, Kaneko K, Konno T, Saito T. Prospective study of superior cluneal nerve disorder as a potential cause of low back pain and leg symptoms. J Orthop Surg Res. 2014;9:139.
Strong EK, Davila JC. The cluneal nerve syndrome; a distinct type of low back pain. Ind Med Surg. 1957;26(9):417–29.
Maigne JY, Lazareth JP, Guerin Surville H, Maigne R. The lateral cutaneous branches of the dorsal rami of the thoraco-lumbar junction. An anatomical study on 37 dissections. Surg Radiol Anat. 1989;11(4):289–93.
Tubbs RS, Levin MR, Loukas M, Potts EA, Cohen-Gadol AA. Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes. J Neurosurg Spine. 2010;13(3):356–9.
Lu J, Ebraheim NA, Huntoon M, Heck BE, Yeasting RA. Anatomic considerations of superior cluneal nerve at posterior iliac crest region. Clin Orthop Relat Res. 1998;347:224–8.
Morimoto D, Isu T, Kim K, Imai T, Yamazaki K, Matsumoto R, Isobe M. Surgical treatment of superior cluneal nerve entrapment neuropathy. J Neurosurg Spine. 2013;19(1):71–5.
Kuniya H, Aota Y, Saito T, Kamiya Y, Funakoshi K, Terayama H, Itoh M. Anatomical study of superior cluneal nerve entrapment. J Neurosurg Spine. 2013;19(1):76–80.
Iwanaga J, Simonds E, Patel M, Oskouian RJ, Tubbs RS. Anatomic study of superior cluneal nerves: application to low back pain and surgical approaches to lumbar vertebrae. World Neurosurg. 2018;116:e766–8.
Iwanaga J, Simonds E, Schumacher M, Oskouian RJ, Tubbs RS. Anatomic study of superior cluneal nerves: revisiting the contribution of lumbar spinal nerves. World Neurosurg. 2019;128:e12–5.
Konno T, Aota Y, Kuniya H, Saito T, Qu N, Hayashi S, Kawata S, Itoh M. Anatomical etiology of “pseudo-sciatica” from superior cluneal nerve entrapment: a laboratory investigation. J Pain Res. 2017;10:2539–45.
Maigne R. Low back pain of thoracolumbar origin. Arch Phys Med Rehabil. 1980;61(9):389–95.
Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976). 1995;20(9):1055–60.
Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine (Phila Pa 1976). 1992;17(12):1474–80.
Kurz LT, Garfin SR, Booth RE Jr. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976). 1989;14(12):1324–31.
Speed S, Sims K, Weinrauch P. Entrapment of the medial branch of the superior cluneal nerve: a previously unrecognized cause of lower back pain in cricket fast bowlers. J Med Cases. 2011;2(3):101–3.
Talu GK, Ozyalcin S, Talu U. Superior cluneal nerve entrapment. Reg Anesth Pain Med. 2000;25(6):648–50.
Maigne JY, Doursounian L. Entrapment neuropathy of the medial superior cluneal nerve. Nineteen cases surgically treated, with a minimum of 2 years’ follow-up. Spine (Phila Pa 1976). 1997;22(10):1156–9.
Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–88.
Leggett LE, Soril LJ, Lorenzetti DL, Noseworthy T, Steadman R, Tiwana S, Clement F. Radiofrequency ablation for chronic low back pain: a systematic review of randomized controlled trials. Pain Res Manag. 2014;19(5):e146-153.
Visnjevac O, Ma F, Abd-Elsayed A. A literature review of dorsal root entry zone complex (DREZC) lesions: integration of translational data for an evolution to more accurate nomenclature. J Pain Res. 2021;14:1.
Costandi S, Garcia-Jacques M, Dews T, Kot M, Wong K, Azer G, Atalla J, Looka M, Nasr E, Mekhail N. Optimal temperature for radiofrequency ablation of lumbar medial branches for treatment of facet-mediated back pain. Pain Pract. 2016;16(8):961–8.
Kapural L, Vrooman B, Sarwar S, Krizanac-Bengez L, Rauck R, Gilmore C, North J, Mekhail N. Radiofrequency intradiscal biacuplasty for treatment of discogenic lower back pain: a 12-month follow-up. Pain Med. 2015;16(3):425–31.
Zheng D, Lamer TJ. Idiopathic cluneal neuralgia successfully treated with radiofrequency nerve ablation: a case report. A&A Pract. 2019;12(10):352–5.
Acknowledgements
Jordan Sam and Asha Hashim, research assistants to Dr. Visnjevac, identified and retrieved relevant references, contributing to the outline of the initial draft of this manuscript.
Funding
No external funding was obtained for this manuscript. All contributions of time and effort by the authors were voluntary without monetary reimbursement.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception or design of the work: OV, MF, TV, AA Data collection: OV, MP Data analysis and interpretation: OV, MP, MF, TV, AA Drafting the article. OV, MP, MF, TV, AA Critical revision of the article: OV, MP, MF, TV, AA Final approval of the version to be published: OV, MP, MF, TV, AA.
Prior Presentation
Prior Presentation at the Canadian Spine Society Annual meeting, 2022.
Disclosures
Authors Mila Pastrak, Frederick Ma, Tanja Visnjevac, Alaa Abd-ElSayed, Ognjen Visnjevac have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the institutional review board (IRB) approval from Veritas IRB (protocol ID: 2021-2584-5272-1) an IRB accredited by the Human Research Accreditation Canada.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Visnjevac, O., Pastrak, M., Ma, F. et al. Radiofrequency Ablation of the Superior Cluneal Nerve: A Novel Minimally Invasive Approach Adopting Recent Anatomic and Neurosurgical Data. Pain Ther 11, 655–665 (2022). https://doi.org/10.1007/s40122-022-00385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00385-x