Abstract
Introduction
The 2019 novel coronavirus (COVID-19) has been recognized as the most severe human infectious disease pandemic in the past century. To enhance our ability to control potential infectious diseases in the future, this study simulated the influence of nucleic acid testing on the transmission of COVID-19 across varied scenarios. Additionally, it assessed the demand for nucleic acid testing under different circumstances, aiming to furnish a decision-making foundation for the implementation of nucleic acid screening measures, the provision of emergency materials, and the allocation of human resources.
Methods
Considering the transmission dynamics of COVID-19 and the preventive measures implemented by countries, we explored three distinct levels of epidemic intensity: community transmission, outbreak, and sporadic cases. Integrating the theory of scenario analysis, we formulated six hypothetical epidemic scenarios, each corresponding to possible occurrences during different phases of the pandemic. We developed an improved SEIR model, validated its accuracy using real-world data, and conducted a comprehensive analysis and prediction of COVID-19 infections under these six scenarios. Simultaneously, we assessed the testing resource requirements associated with each scenario.
Results
We compared the predicted number of infections simulated by the modified SEIR model with the actual reported cases in Israel to validate the model. The root mean square error (RMSE) was 350.09, and the R-squared (R2) was 0.99, indicating a well-fitted model. Scenario 4 demonstrated the most effective prevention and control outcomes. Strengthening non-pharmaceutical interventions and increasing nucleic acid testing frequency, even under low testing capacity, resulted in a delayed epidemic peak by 78 days. The proportion of undetected cases decreased from 77.83% to 31.21%, and the overall testing demand significantly decreased, meeting maximum demand even with low testing capacity. The initiation of testing influenced case detection probability. Under high testing capacity, increasing testing frequency elevated the detection rate from 36.40% to 77.83%. Nucleic acid screening proved effective in reducing the demand for testing resources under diverse epidemic prevention and control strategies. While effective interventions and nucleic acid screening measures substantially diminished the demand for testing-related resources, varying degrees of insufficient testing capacity may still persist.
Conclusions
The nucleic acid detection strategy proves effective in promptly identifying and isolating infected individuals, thereby mitigating the infection peak and extending the time to peak. In situations with constrained testing capacity, implementing more stringent measures can notably decrease the number of infections and alleviate resource demands. The improved SEIR model demonstrates proficiency in predicting both reported and unreported cases, offering valuable insights for future infection risk assessments. Rapid evaluations of testing requirements across diverse scenarios can aptly address resource limitations in specific regions, offering substantial evidence for the formulation of future infectious disease testing strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nucleic acid testing, as a crucial component of precision prevention and control, plays a pivotal role in promptly screening and isolating infected individuals, thereby contributing to a reduction in the overall number of infections. |
Many health facilities in various regions have faced challenges due to resource constraints during the battle against the outbreak. Testing capacity stands as a vital metric, reflecting the maximum number of nucleic acid tests achievable in a region under varying degrees of resource constraints. This capacity significantly influences the sustainability of nucleic acid testing strategies. Upon reviewing the trajectory of infectious disease pandemics, it has been observed that numerous regions in China faced shortages in resources for nucleic acid testing. |
This study considers the willingness of residents to choose nucleic acid testing and the accuracy of nucleic acid laboratory testing techniques, aiming to develop a mathematical model to predict the number of infections in real situations. According to the predicted number of infections, the demand for nucleic acid testing and the effectiveness of prevention and control strategies were evaluated. |
In different scenarios with the same initial infected person, the scale of infection after 200 days of epidemic simulation follows the order scenario 4, scenario 3, scenario 5, scenario 2, scenario 6, and scenario 1. In scenario 4, with improved nucleic acid testing capacity compared to scenario 3, the number of undetected cases and test-negative cases decreased, and the proportion of undetected cases was 22.14%. |
In situations with constrained testing capacity, implementing more stringent measures can notably decrease the number of infections and alleviate resource demands. The improved SEIR model demonstrates proficiency in predicting both reported and unreported cases, offering valuable insights for future infection risk assessments. |
Introduction
Infectious diseases are one of the most important threats to human survival and health and hinder social and economic development. The 2019 novel coronavirus (COVID-19) is the most serious human infectious disease pandemic in the past century [1]. To curb the transmission of SARS-CoV-2, countries have implemented a diverse array of prevention and control measures, primarily encompassing non-pharmaceutical interventions and vaccination efforts [2]. Following the onset of COVID-19 in early 2020, China swiftly implemented the initial lockdown and control measures. While these actions effectively curtailed the epidemic’s spread, they also had significant repercussions on social production and people’s daily lives. Consequently, starting in April 2020, China initiated a shift from comprehensive prevention and control to precision-based strategies, aiming to strike a balance between curbing the virus’s impact and minimizing disruptions to societal functions [3]. Nucleic acid testing, as a crucial component of precision prevention and control, plays a pivotal role in promptly screening and isolating infected individuals, thereby contributing to a reduction in the overall number of infections [4, 5].
However, the surge in asymptomatic infections during the circulation of the Omicron strain has posed challenges to the swift identification of infected individuals through rapid screening [6]. Furthermore, the impact of false negative results in the laboratory testing process and the ongoing mutation of the strain underscore the need to intensify detection efforts within the population to promptly identify infected individuals. Many health facilities in various regions have faced challenges due to resource constraints during the battle against the outbreak [7]. Testing capacity stands as a vital metric, reflecting the maximum number of nucleic acid tests achievable in a region under varying degrees of resource constraints. This capacity significantly influences the sustainability of nucleic acid testing strategies [8].
Upon reviewing the trajectory of infectious disease pandemics, it has been observed that numerous regions in China faced shortages in resources for nucleic acid testing. Conducting a needs assessment emerges as an effective strategy to streamline resource allocation. By gauging anticipated medical and health requirements, a timely resource allocation plan can be devised to prevent the misuse or scarcity of resources [9]. Nevertheless, the fluctuation of virus strains and the adjustment of prevention and control strategies will impact the incidence of infections, subsequently influencing the demand for resources [10]. Therefore, the demand for nucleic acid testing under varying circumstances remains uncertain, with the question of whether it will surpass nucleic acid testing capacity is yet to be determined.
Previous studies have shown that the SEIR model can effectively simulate the development trend of the COVID-19 epidemic, evaluate the demand for laboratory testing resources, and optimizes resource allocation [11,12,13]. A study conducted in the UK utilized the SEIR-D model to forecast the number of infections in local areas, gauge healthcare requirements, and anticipate needs and isolation capacity within regional hospitals [14]. Cui et al. [15] employed an improved SEIR model to accurately simulate and predict the transmission dynamics of COVID-19 in two low-income countries, namely Kazakhstan and Pakistan. Their findings offer valuable insights, serving as a reference for low-income countries in formulating effective prevention and control strategies. In India, a study utilized the SEIR model in conjunction with sociodemographic variables to guide the development of a prioritized testing strategy for infectious disease control. This approach not only reduced the utilization of testing resources but also minimized the scale of infections and shortened outbreak durations compared to traditional testing models [16]. However, existing studies frequently rely on the number of infections to validate models, often overlooking the impact of the false negative rate in virus detection. This oversight may result in an imprecise modeling of COVID-19 transmission dynamics based solely on the reported “cases.” Moreover, the emphasis tends to be on evaluating the effectiveness of prevention and control measures, such as vaccines and quarantine, with less attention given to resource constraints.
In this study, we consider the false negative rate of SARS-CoV-2 testing and address the selection bias resulting from testing priority, acknowledging the potential bias in validating the model solely based on reported cases. Consequently, we established an infectious disease model based on testing outcomes to analyze the effects of varying testing resources and the intensity of prevention and control measures on the epidemic trajectory. The aim is to offer insights and guidance for the development of resource allocation plans in future public health emergencies.
Methods
This study considers the willingness of residents to choose nucleic acid testing and the accuracy of nucleic acid laboratory testing techniques, aiming to develop a mathematical model to predict the number of infections in real situations. According to the predicted number of infections, the demand for nucleic acid testing and the effectiveness of prevention and control strategies were evaluated.
Data Collection
To enhance the reliability of model simulation results and considering the backdrop of the epidemic, Haifa, Israel, characterized by high vaccine coverage and a relatively severe outbreak, was chosen as the focal point of this research. A comparative analysis was conducted by juxtaposing the predicted number of individuals within a specific period with the actual count. Real-time data, including confirmed cases, vaccinated individuals, and detected cases, from December 25, 2021 to April 29, 2022, were sourced from the official website of the Israeli Ministry of Health. Following the determination of model parameters, the infectious disease model’s validity was assessed by contrasting simulation outcomes with actual data (Table 1). Ethics approval and consent to participate in this study are not applicable.
Establishment of Infectious Disease Model
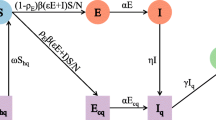
We formulated an improved SEIR model to simulate the epidemic’s developmental trajectory (Fig. 1). Unlike the traditional SEIR model, which categorizes the study population into four states (S, E, I, and R) based on different stages of the infectious disease course, and assumes the total population in the area as N (i.e., N = S + E + I + R), our improved model introduces considerations for the protective impact of vaccines and virus detection measures. Specifically, we modified the SEIR model to account for whether testing was conducted and whether the infected person was detected. Compartment I was subdivided into untested infected individuals (U), tested positive infected individuals (P), and tested negative infected individuals (F).
Model assumptions:
-
1.
The floating population in the study area remained in equilibrium throughout the research period, with the total population remaining constant. This implies that the birth rate equals the mortality rate, and the immigration rate equals the emigration rate.
-
2.
As a result of the continuous mutation of the virus, immunity acquired through previous infection does not shield susceptible individuals from new strain infections. Therefore, the population is considered generally susceptible, and all individuals are at risk of infection.
-
3.
Patients who recovered from COVID-19 attain temporary immunity and are not susceptible to reinfection.
-
4.
The level of vaccine protection does not diminish over the study period after vaccination.
Differential equation:
Definition of Nucleic Acid Testing Capacity and Demand
Testing capacity was stipulated as 50% (125,000) of the total population tested daily with high testing capacity and 25% (62,500) of the total population tested daily with low testing capacity. The demand for nucleic acid testing primarily comprises the quantity of tests utilized and the potential testing demand. “Used test quantity” refers to the total number of individuals tested throughout the outbreak. The “potential demand for testing” encompasses individuals requiring retesting because of exposure or those who have not been identified as infected.
Scenario Setting
To assess the trajectory of the COVID-19 epidemic under various testing capabilities, three epidemic intensities were established: community transmission, outbreak, and sporadic. The transmission of COVID-19 was simulated under different testing strategies. Drawing on scenario construction theory, epidemic scenarios with distinct testing capabilities and strategies were crafted through expert consultation and group discussion. The retrospective summary of the epidemic’s development characteristics both domestically and internationally during scenario construction is detailed as follows:
Scenario 1: Baseline scenario, assuming no intervention is implemented, and the initial exposure is 5% of the total population, portraying a natural progression independent of testing capacity.
Scenario 2: Disease outbreak scenario with numerous imported cases at the initial stage. The initial exposure population was set at 2% of the total population. As a result of low COVID-19 vaccination coverage in the initial epidemic stage, the government did not rigorously implement non-pharmaceutical prevention and control measures (50%). Additionally, nucleic acid detection capacity in this region was low, with a 3-day duration from sampling to result reporting and a high false-negative rate. The initial input quantitation was set at 10 cases, including 5 undetected infected patients, 2 patients testing positive, and 3 patients testing undetected (Table 2). All individuals were infectious with no reported deaths or recoveries.
Scenario 3: Building upon scenario 2, it was a community-based cluster outbreak with a 20% reduction in the initial exposed population. The local government prioritized prevention and control, implementing strict non-pharmaceutical measures, and tested infected individuals 1 day after detection. The remaining elements were consistent with scenario 2.
Scenario 4: Based on scenario 3, this scenario increased the initial exposed population by 50%. The vaccination level of residents in the area was high, and the testing ability was robust, allowing quick reporting of test results and minimizing the probability of false negatives.
Scenario 5: Building upon scenario 4, the initial exposed population was reduced by 50%, residents received voluntary testing, and all other elements remained consistent with scenario 4.
Scenario 6: Building upon scenario 2, the initial exposed population increased by 50%. Considering social and economic benefits, the government did not enforce strict prevention and control measures, and residents underwent voluntary testing. However, the vaccination level remained high, and other factors were consistent with scenario 2.
Model Calibration and Verification
Israel boasts one of the highest vaccination rates globally and has been significantly impacted by the current Omicron outbreak. With 1892 cases per million people during the Omicron epidemic, Israel leads the world in case numbers, surpassing second-place Mongolia’s 1119 cases. Given the relatively comprehensive data on confirmed cases reported in Israel compared to other regions during the same period, this study validated the model by comparing the predicted number of infections simulated by the improved SEIR model with the actual number of reported cases in Israel. The root mean square error (RMSE) was 350.09, and the R-squared (R2) was 0.99, indicating a robust fitting effect. The simulated curve closely aligned with the epidemic data from Haifa City, Israel, as retrieved from the official source (https://data.gov.il/dataset/covid-19, December 20, 2023) (Fig. 2).
Results
On the basis of the improved SEIR model, we simulated the trajectory of infected cases in various outbreak scenarios under nucleic acid detection measures and estimated the nucleic acid detection quantity based on the model simulation results. This information is valuable for adjusting strategies according to the available detection capacity.
In different scenarios with the same initial infected person, the scale of infection after 200 days of epidemic simulation follows this order: scenario 4, scenario 3, scenario 5, scenario 2, scenario 6, scenario 1 (Table 3).
Scenario 1
In the scenario without any interventions, the outbreak peaks at approximately 30 days, with 143,195 people infected, constituting 57.28% of the total population (Fig. 3a) All the infected cases were untested (Fig. 4a). As a result of the lack of intervention, there is a potential demand of 187,148, far exceeding the set nucleic acid detection capacity (Table 4).
Scenario 2
Under a scenario with relaxed containment measures and low testing capacity, the epidemic peaks at about 44 days, with 115,419 people infected (Fig. 3b) Among them, 83,518 are undiagnosed cases, 25,589 are positive cases, and 6312 are negative cases (Fig. 4b). Undetected cases account for 77.83% of the total cases. The total demand reaches a peak of 121,201 on the 41st day, surpassing the capacity when testing is low (Table 4).
Scenario 3
In a scenario with low testing capacity but strengthened non-pharmaceutical prevention and control measures, the epidemic peaks at 122 days, with 21,096 infected people (Fig. 3c) Among them, 2875 are undetected, 14,511 are positive, and 3710 are negative. Undetected cases account for 31.21% of the total cases (Fig. 4c). The total demand peaks at 11,739 on the 115th day, and the demand can be met even with low detection capacity (Table 4).
Scenario 4
With increased testing capacity, intensified non-pharmaceutical prevention and control measures, and vaccination, the epidemic peaks at 108 days, with 19,303 infected people (Fig. 3d). Among them, 2657 are undetected, 15,024 are positive, and 1622 are negative. Undetected cases account for 22.17% of the total cases (Fig. 4d). The total demand peaks at 12,884 on the 97th day, and both high and low detection capabilities can meet the demand at this time (Table 4).
Scenario 5
Under strict prevention and control measures and robust testing capacity, the epidemic peaks at 125 days, with 30,942 infected people (Fig. 3e). Among them, 18,468 are undetected, 11,262 are positive, and 1212 are negative. Undetected cases account for 63.60% of the total cases (Fig. 4e). The total demand peaks at 32,537 on the 119th day, and both high and low detection capabilities can meet the demand at this time (Table 4).
Scenario 6
In a scenario of high vaccination efficiency and low testing capacity with relaxed prevention and control measures, the epidemic peaks at about 39 days, with 119,103 infected people (Fig. 3f). Among them, 102,870 are undetected, 13,018 are positive, and 3215 are negative. Undetected cases account for 89.07% of the total cases (Fig. 4f). The total demand peaks at 134,320 on the 36th day, and neither high nor low detection capabilities can meet the demand at this time (Table 4).
Comparative Analysis Result
There were significant differences in the total demand for testing under different scenarios (Fig. 5).
Compared with no prevention and control measures, relatively lenient prevention and control measures can reduce the infection peak and prolong the epidemic’s peak time. Building upon the implementation of scenario 1, scenario 2 introduced non-pharmaceutical interventions, vaccination, nucleic acid testing, and other measures, resulting in a reduction of 27,776 infections and 65,947 in total testing demand. By intensifying non-pharmaceutical interventions and increasing the frequency of nucleic acid testing in scenario 3, even under low testing capacity, the epidemic’s peak time was delayed by 78 days. The proportion of undetected cases decreased from 77.83% to 31.21%, and the total demand for testing was significantly reduced, meeting the maximum demand under low testing capacity.
In scenario 4, with improved nucleic acid testing capacity compared to scenario 3, the number of undetected cases and test-negative cases decreased, and the proportion of undetected cases was 22.17%. However, the total demand for testing increased in this scenario. Scenario 5 transitioned from universal testing to voluntary testing on the basis of scenario 4, resulting in a 44% decrease in residents’ willingness to test. Consequently, the number of infected patients increased by 11,640, the proportion of undetected cases surged to 63.60%, and the total demand rose by 2.5 times, surpassing the set capacity of nucleic acid testing.
Under scenario 6, where vaccination coverage increased and residents’ willingness to test decreased on the basis of scenario 2, the number of infected people increased by 3685, the proportion of undetected cases rose by 11.24%, and the total demand for nucleic acid testing increased by 13,119. This analysis underscores the intricate dynamics between prevention and control measures, testing strategies, and their collective impact on epidemic outcomes.
Discussion
Based on the transmission characteristics of COVID-19, the traditional SEIR model was improved in this study. Information and data published on official Israeli websites during the outbreak were collected to construct a dynamic model of the infectious disease considering detection measures. This model can provide some theoretical basis and support for the prevention and control of the epidemic. We also summarized the prevention and control experience and laboratory resource requirements of China and other countries during the COVID-19 epidemic, focusing on the impact of nucleic acid detection capacity on the prevention and control of the epidemic. This study used a transmission dynamics model to simulate the development trend of the epidemic in different scenarios, and comprehensively analyzed the demand for medical and health resources under different circumstances, so as to provide reference for emergency preparedness and prevention and control strategy adjustment of medical resources under the epidemic. Guidance on resource input and allocation of control materials is also provided.
As a result of infectious diseases being affected by many factors in real life, it is difficult to analyze the development of epidemics in different scenarios in the future. However, the scenario simulation method provides new ideas for the analysis of the development trend of COVID-19 and the decision-making of prevention and control [21, 22]. Scenario simulation is a setting method that mimics the real-life scene. Scenario simulation can set up a scene to carry out research by analyzing the determined conditions, limit the uncertainty of the development of things, predict the possible impact of various scenarios in the future, and provide guidance for the reality of the situation. Israel, which has one of the highest vaccination rates globally, is the country hardest hit by the current outbreak [23]. Using this data to validate our modified SEIR model, which takes into account the effect of vaccination, we found that the results fit well, with Israel having 1892 confirmed cases per million population on September 1, 2022, which was the highest in the world and well ahead of Mongolia, which had 1119 cases. The results showed that when the national testing strategy changed to voluntary testing, the willingness of residents to test decreased by 44%. The number of infected patients increased by 11,640, the proportion of undetected cases increased to 63.60%, and the total demand increased by 2.5 times, which was far beyond the set nucleic acid testing capacity. It is necessary to strengthen the intensity of interventions and increase testing in the early stage of the epidemic to help control the demand for testing and keep it within the capacity.
We estimated the demand for nucleic acid testing by quantifying the change in the number of infections during the COVID-19 epidemic, explored how to optimize the nucleic acid testing strategy with the nucleic acid testing capacity as the constraint, and determined the best nucleic acid testing implementation plan to provide guidance for the use and deployment of resources. China’s previous epidemic prevention experience shows that once a regional epidemic occurs, it is necessary to organize a large-scale nucleic acid screening immediately to find the infected patients in time and immediately isolate the positive cases. Active detection can be an effective strategy to prevent the spread of SARS-CoV-2 [24]. Our findings suggest that increased frequency of nucleic acid testing with increased intensity of non-pharmaceutical interventions may delay the peak of the epidemic by 78 days under low nucleic acid testing capacity. Xiang et al. showed that the implementation of traffic control policies reduced the peak number of infected people in Changsha by 66.03%, and the peak period was delayed by 58 days [25]. Early large-scale testing and strict prevention and control measures can effectively reduce the scale of infection and the potential demand for nucleic acid testing. The results of a Brazilian study suggest that the relatively early adoption of quarantine in the state of Sao Paulo, compared to the lockdown in Spain, resulted in a prolonged duration of the first wave of the outbreak and a delay in its peak [26]. This is consistent with the results of our study.
We consider the initiation and false negative rate of nucleic acid testing, and the improvement of SEIR model is beneficial to distinguish the infected persons who have been detected from those who have not been detected, which is beneficial to estimate the level of future risk. Bhaduri et al. [27] simulated and estimated the number of undetected infections and deaths in India by modifying the SEIR model. In addition, nucleic acid screening requires significant time, resources, and personnel, and as the risk of infection changes in different regions, governments may simplify their testing models, so it is critical to estimate the need for nucleic acid testing in different scenarios. Studies have shown that costs lost as a result of lockdowns can be avoided through large-scale nucleic acid testing [28]. However, the key to the implementation of nucleic acid testing measures lies in the testing capacity. It is helpful to optimize the allocation of testing resources by estimating the testing needs in different situations. Sainz-Pardo et al. [29] introduced a heuristic to minimize the spread of COVID-19 by planning an effective distribution of tests in a population in an area over time. Some studies have pointed out that in the case of limited medical resources, the optimal allocation of resources in multiple regions depends on the state of the whole region, and a region may change from limited testing resources to having enough testing resources [30]. Therefore, it is necessary to accurately and rapidly assess the demand for nucleic acid testing in different situations.
This study still has some limitations. Firstly, most of the published literature was referred to in terms of parameter selection for the infectious disease model. As a result of the differences in model parameters between regions and periods, further validation with empirical data is lacking. Second, we ignored the heterogeneity of incidence in different regions and did not consider socioeconomic factors such as age and underlying diseases that are related to the risk of COVID-19 infection. Third, this study focused on the impact of nucleic acid screening measures on the development of the epidemic and only involved nucleic acid testing resources. Subsequent studies can comprehensively evaluate the resources needed after the outbreak, including beds, drugs, vaccines, etc. Future studies should collect more case data and epidemiological data, combined with socioeconomic factors, and improve the parameter settings and values to improve the accuracy of the model. In addition, this demand estimation method can also be used to solve the problem of resource allocation optimization in specific places such as communities, schools, and hospitals.
Conclusions
The implementation of strict nucleic acid testing strategy can reduce the infection peak and delay the peak time. However, when testing capacity is limited, more stringent prevention and control measures can reduce the number of infections and the need for resources. The improved SEIR model can better predict reported and unreported cases and make recommendations for future infection risk. Rapid assessment of testing needs in different situations can solve the problem of limited resources in some areas through resource allocation and provide reference for the prevention and control of infectious diseases in the future.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Wu Q, Dong S, Li X, et al. Effects of COVID-19 non-pharmacological interventions on dengue infection: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2022;12:892508.
Zhu N, Tan W. Control and challenge of COVID-19: lessons from China’s experience. Am J Physiol Lung Cell Mol Physiol. 2021;321(5):L958–9.
Zhang N, Shi T, Zhong H, et al. COVID-19 prevention and control public health strategies in Shanghai. China J Public Health Manag Pract. 2020;26(4):334–44.
Zhu W, Zhu Y, Wen Z, et al. Quantitative assessment of the effects of massive nucleic acid testing in controlling a COVID-19 outbreak. BMC Infect Dis. 2022;22(1):845.
Gao Q, Shang WP, Jing MX. Effect of nucleic acid screening measures on COVID-19 transmission in cities of different scales and assessment of related testing resource demands-evidence from China. Int J Environ Res Public Health. 2022;19(20):13343.
Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327(6):583–4.
Olalekan A, Iwalokun B, Akinloye OM, et al. COVID-19 rapid diagnostic test could contain transmission in low- and middle-income countries. Afr J Lab Med. 2020;9(1):1255.
Kebede A, Lanyero B, Beyene B, et al. Expanding molecular diagnostic capacity for COVID-19 in Ethiopia: operational implications, challenges and lessons learnt. Pan Afr Med J. 2021;38:68.
Yudkin JS, Hodes RM, Sandler-Loeff A, et al. Needs assessment and best practices for digital trainings for health professionals in Ethiopia using the RE-AIM framework: COVID-19, case study. Disaster Med Public Health Prep. 2022;17:e292.
Gardner Yelton SE, McCaw JM, Reuland CJ, et al. Evolution of a bidirectional pediatric critical care educational partnership in a resource-limited setting. Front Pediatr. 2021;9:738975.
Semenova Y, Pivina L, Khismetova Z, et al. Anticipating the need for healthcare resources following the escalation of the COVID-19 outbreak in the Republic of Kazakhstan. J Prev Med Public Health. 2020;53(6):387–96.
Inthamoussou FA, Valenciaga F, Núñez S, et al. Extended SEIR model for health policies assessment against the COVID-19 pandemic: the case of Argentina. J Healthc Inform Res. 2022;6(1):91–111.
Qiu T, Xiao H, Brusic V. Estimating the effects of public health measures by SEIR(MH) model of COVID-19 epidemic in local geographic areas. Front Public Health. 2021;9: 728525.
Campillo-Funollet E, Van Yperen J, Allman P, et al. Predicting and forecasting the impact of local outbreaks of COVID-19: use of SEIR-D quantitative epidemiological modelling for healthcare demand and capacity. Int J Epidemiol. 2021;50(4):1103–13.
Cui Q, Shi Z, Yimamaidi D, et al. Dynamic variations in COVID-19 with the SARS-CoV-2 Omicron variant in Kazakhstan and Pakistan. Infect Dis Poverty. 2023;12(1):18.
Evans MV, Ramiadantsoa T, Kauffman K, et al. Socio-demographic variables can guide prioritized testing strategies for epidemic control in resource-limited contexts. J Infect Dis. 2023;228(9):1189–97.
Li XN, Huang Y, Wang W, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021;10(1):1751–9.
Filchakova O, Dossym D, Ilyas A, et al. Review of COVID-19 testing and diagnostic methods. Talanta. 2022;244:123409.
Xin H, Li Y, Wu P, et al. Estimating the latent period of coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2022;74(9):1678–81.
Paul S, Lorin E. Estimation of COVID-19 recovery and decease periods in Canada using delay model. Sci Rep. 2021;11(1):23763.
Shen S, Li W, Wei H, et al. A chess and card room-induced COVID-19 outbreak and its agent-based simulation in Yangzhou. China Front Public Health. 2022;10:915716.
Wei Y, Wang J, Song W, et al. Spread of COVID-19 in China: analysis from a city-based epidemic and mobility model. Cities. 2021;110:103010.
Wang Q, Li S, Li R, et al. Underestimated impact of the COVID-19 on carbon emission reduction in developing countries - a novel assessment based on scenario analysis. Environ Res. 2022;204(Pt A):111990.
Ding D, Zhang R. China’s COVID-19 control strategy and its impact on the global pandemic. Front Public Health. 2022;10:857003.
Xiang W, Chen L, Peng Q, et al. How effective is a traffic control policy in blocking the spread of COVID-19? A case study of Changsha, China. Int J Environ Res Public Health. 2022;19(13):7884.
Perez-Saez J, Lee EC, Wada NI, et al. Effect of non-pharmaceutical interventions in the early phase of the COVID-19 epidemic in Saudi Arabia. PLOS Glob Public Health. 2022;2(5):e0000237.
Babu GR, Ray D, Bhaduri R, et al. COVID-19 pandemic in India: through the lens of modeling. Glob Health Sci Pract. 2021;9(2):220–8.
Candel FJ, Viayna E, Callejo D, et al. Social restrictions versus testing campaigns in the COVID-19 crisis: a predictive model based on the Spanish case. Viruses. 2021;13(5):917.
Sainz-Pardo JL, Valero J. COVID-19 and other viruses: holding back its spreading by massive testing. Expert Syst Appl. 2021;186:115710.
Ren J, Liu M, Liu Y, et al. Optimal resource allocation with spatiotemporal transmission discovery for effective disease control. Infect Dis Poverty. 2022;11(1):34.
Medical Writing, Editorial, and Other Assistance.
We used ChatGPT19.0 to modify the English language and grammar of the article.
Funding
Funding for the study and the journal’s Rapid Service fee was provided by the Xinjiang Production and Construction Corps Scientific and Technological Research Project (2021AB034).
Author information
Authors and Affiliations
Contributions
Conceptualization, Yu-Yuan Wang and Ming-Xia Jing; methodology, Yu-Yuan Wang and Jia-Lin Sun; formal analysis, Yu-Yuan Wang and Wei-Wen Zhang; data curation, Ze-Xi Lu; writing—original draft preparation, Yu-Yuan Wang; writing—review and editing, Jia-Lin Sun and Yu-Yuan Wang; supervision, Ming-Xia Jing; project administration, Jia-Lin Sun and Ming-Xia Jing; funding acquisition, Ming-Xia Jing. All authors contributed to interpreting results and editing the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Yu-Yuan Wang, Wei-Wen Zhang, Ze-xi Lu, Jia-lin Sun and Ming-xia Jing have nothing to disclose. All named authors confirm that they have no conflicts of interest to declare.
Ethical Approval
Ethics approval and consent to participate in this study are not applicable. All authors gave final approval for publication. No other consent was required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, YY., Zhang, WW., Lu, Zx. et al. Evaluating the Demand for Nucleic Acid Testing in Different Scenarios of COVID-19 Transmission: A Simulation Study. Infect Dis Ther 13, 813–826 (2024). https://doi.org/10.1007/s40121-024-00954-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00954-x