Abstract
Introduction
The risk of herpes zoster (HZ) is associated with a decline in immune system function, linked to aging and/or immunocompromising or immunosuppressive diseases or therapies. In this post hoc analysis we describe the incidence of HZ, rash characteristics, and burden of HZ pain in immunocompetent adults ≥ 50 years of age (YOA) and in hematopoietic stem cell transplantation (HSCT) recipients ≥ 18 YOA.
Methods
ZOE-50 (NCT01165177), ZOE-70 (NCT01165229), and ZOE-HSCT (NCT01610414) were phase III, observer-blind, placebo-controlled, randomized studies conducted in immunocompetent adults ≥ 50 YOA and ≥ 70 YOA; and in HSCT recipients ≥ 18 YOA, respectively. A similar methodology for study design, case definition, and data collection were applied in all three studies. The participants received either two doses of the adjuvanted recombinant zoster vaccine or placebo, 1–2 months apart. This analysis focuses on all confirmed HZ cases from the placebo groups of the three studies. HZ pain and interference with activities of daily living were assessed using the Zoster Brief Pain Inventory instrument.
Results
Overall, 280, 240, and 172 placebo participants with an HZ confirmed episode aged ≥ 50, ≥ 70, and ≥ 18 YOA were included in the ZOE-50, ZOE-70, and ZOE-HSCT analyses, respectively. The incidence of HZ was 9.1/1000 person-years in both the ZOE-50 and ZOE-70 placebo groups and 95.6/1000 person-years in the ZOE-HSCT study placebo group. In the three studies, most individuals with HZ had severe pain, with approximately 90% of individuals reporting clinically significant pain. An estimated 12.3%, 16.9%, and 21.8% of patients in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively, developed post-herpetic neuralgia.
Conclusion
The incidence and burden of HZ is high in immunocompetent adults aged ≥ 50 YOA and even more so in HSCT recipients aged ≥ 18 YOA.
Graphical Abstract

Plain language summary
-
Shingles is a viral disease caused by the reactivation of the varicella virus in adults. Symptoms include a painful rash that shows up on the side of the body.
-
Researchers conducted three large phase III clinical trials in immunocompetent individuals aged 50 years and older and in adult immunocompromised patients using similar methodologies for study design, case definition, data collection, and analysis in these populations.
-
This post-hoc analysis from these three large phase III clinical trials analyzed the incidence of shingles and the rash and pain characteristics in adults who received placebo instead of vaccination with recombinant zoster vaccine.
-
Immunocompetent individuals aged 50 years and older showed a high incidence of shingles, and this was even more pronounced in immunocompromised adults aged 18 years and older.
-
Most adults with shingles had severe pain.
-
Immunocompromised patients more frequently had long-lasting pain (post-herpetic neuralgia).
-
Adults aged 50 years and older most frequently had a rash on the upper trunk while immunocompromised patients most frequently had a rash on the lower trunk.
-
This study showed the high impact of shingles, especially in immunocompromised patients.
-
Vaccination is recommended in older adults (more than 50 years) and in adult immunocompromised populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There are very few prospective studies with robust sample sizes evaluating both clinical and patient-reported outcomes in immunocompromised individuals with herpes zoster. |
Three phase III randomized trials were conducted to assess the efficacy of the herpes zoster vaccine in two adult populations (subjects aged ≥ 50 years and ≥ 70 years), and one immunocompromised population (HSCT recipients aged ≥ 18 years). |
This study analyzed data from the unvaccinated groups of the three studies and presented the incidence of herpes zoster and the rash and pain characteristics among the three populations. |
What was learned from the study? |
Overall, the incidence and burden of herpes zoster was high in unvaccinated immunocompetent individuals aged ≥ 50 years and even more so in unvaccinated immunocompromised adults. |
Most older adults who developed HZ reported severe pain, while immunocompromised individuals were at a greater risk of developing post-herpetic neuralgia. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.20481465.
Introduction
Herpes zoster (HZ) typically manifests as a unilateral, painful dermatomal rash, also known as shingles [1]. An HZ episode can be divided into four phases: prodromal, acute, subacute, and chronic [1]. The prodromal phase starts 1–5 days before the onset of HZ rash in most cases, with patients experiencing altered sensation in the affected dermatome, such as pain, tingling, and/or itching; systemic symptoms such as malaise; or headache and/or photophobia, in the case of trigeminal nerve involvement [2,3,4]. The acute phase begins with a red, maculopapular, dermatomal rash that often consists of clusters of erythematous papules, which may evolve into vesicles or pustules, ulcers, and crusts, and generally resolve after 2–3 weeks. During the acute (≤ 30 days post rash onset) and subacute (31–89 days post rash onset) phases of HZ, patients may experience complications such as HZ ophthalmicus, which may lead to vision loss in rare cases; hearing loss; lesion scarring; cardiovascular and stroke events; and/or neurological complications including myelitis, chronic encephalitis, ventriculitis, meningoencephalitis, and cranial palsies [5, 6]. Most HZ cases resolve during the acute/subacute phases, but around 30% of patients with HZ develop postherpetic neuralgia (PHN), a chronic condition of debilitating pain that may last for months or even years and is very difficult to treat [3, 7,8,9].
In a study involving 1005 individual patient-reported outcomes from six European countries, HZ pain was described as “burning” (56%), “itching” (49%), “stabbing” (40%), “shooting” (21%), “throbbing” (11%), “electric shocks” (10%), and “painful cold” (7%) [10]. The pain and discomfort (including allodynia and intense pruritus) experienced during an HZ episode may substantially reduce patients’ health-related quality of life, impacting their mental health and ability to perform activities of daily living (ADLs) [1, 11, 12].
The risk of HZ is associated with a decline in immune system function, especially varicella-zoster virus-specific T cell responses [13], linked to aging and/or in individuals who are immunocompromised or immunosuppressed as a result of disease or therapy [14]. One in three people in the USA will develop shingles in their lifetime resulting in an estimated one million new cases annually [5]. Recombinant zoster vaccine (RZV, Shingrix, GSK) is an adjuvanted subunit vaccine that substantially reduces the risk of HZ in older and immunocompromised populations [15,16,17]. Three multinational phase III randomized trials were conducted using similar methods to assess the efficacy of RZV in preventing HZ in two adult populations (ZOE-50 study [NCT01165177] included participants ≥ 50 years of age (YOA) and ZOE-70 [NCT01165229] included participants ≥ 70 YOA), and one immunocompromised population (ZOE-HSCT study [NCT01610414] in autologous hematopoietic stem cell transplantation (HSCT) recipients ≥ 18 YOA) [15,16,17].
In this manuscript, using data from the ZOE studies, we present results from the placebo groups showing the natural history of disease, i.e., in the absence of vaccination. We describe the incidence of HZ, rash characteristics, and burden of HZ pain in immunocompetent and immunocompromised patients.
Methods
Study Design
ZOE-50 (NCT01165177), ZOE-70 (NCT01165229), and ZOE-HSCT (NCT01610414) were phase III, observer-blind, placebo-controlled, randomized studies conducted in immunocompetent adults ≥ 50 YOA and ≥ 70 YOA; and in HSCT recipients aged ≥ 18 YOA, respectively. A similar methodology for study design, case definition, and data collection was applied in all three studies. However, one major difference between the studies was that the ZOE-50/70 studies excluded participants with any confirmed or suspected immunosuppressive or immunodeficient condition resulting from disease (e.g., malignancy, HIV infection) and/or participants receiving immunosuppressive/cytotoxic therapy (e.g., medications used during cancer chemotherapy, organ transplantation, or to treat autoimmune disorders)”. For the ZOE-HSCT study detailed data on conditioning, maintenance, and immunosuppressive treatments are provided in Supplemental text eTable2 in the publication by Bastidas et al. [17].
In all three studies, the participants received two doses of the adjuvanted RZV or placebo, 1–2 months apart. This analysis was done involving all participants from the placebo groups of the three studies with a confirmed episode of HZ. Further details on the study design are provided in the articles presenting the efficacy and safety results of the individual trials [15,16,17].
Outcome Measures
The investigator or his delegate performed a clinical examination when the participant visited the study site for the first evaluation of the suspected case of HZ. The information was recorded in the case report form, such as date of onset of pain and rash, location and nature of HZ lesions, concomitant medications for HZ treatment, or treatment of any HZ-related complications.
Participants with suspected HZ were asked to complete the Zoster Brief Pain Inventory (ZBPI) daily for 28 days after rash onset and then weekly for (1) a minimum of 90 days after rash onset or until the participant had been pain-free for four consecutive weeks, and (2) a maximum of 182 days [18, 19]. The ZBPI asks the participant to rate four categories of pain (least, worst, and average “in the last 24 h,” in addition to “right now”) on 11-point Likert-type scales (0–10, with 10 signifying the worst imaginable pain). The ZBPI questionnaire also assesses the degree to which the HZ pain interferes with seven ADLs: general activity, mood, walking ability, work, relations with others, sleep, and enjoyment of life. These are all rated on 11-point Likert-type scales with 0 signifying “does not interfere” and 10 “completely interferes.” A summary ADL score is calculated by averaging the scores for the seven activities [18].
Duration of clinically significant pain was estimated using the Kaplan–Meier method. The duration of clinically significant pain was set to 0 for patients with no pain score ≥ 3. Participants without a documented 4-week clinically significant pain-free period were censored at the last date of assessment.
For each case of HZ, the maximal ZBPI “worst pain” score and the maximal ZBPI ADL scores during the HZ episode were calculated. Any pain was defined as a ZBPI “worst pain” score ≥ 1, clinically significant pain as a score ≥ 3, and severe pain as a score ≥ 7. A combined measure of pain intensity and duration was calculated by the area under the curve (AUC) method [18, 19]. The ZBPI severity of illness scores were calculated as the AUC from the day of rash onset until day 182.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
In total 692 placebo HZ cases were included in the analysis from the ZOE studies with 280, 240, and 172 cases occurring in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively. The patient demographics by study group are presented in Table 1. As per the study design, the mean age was 63.1 YOA in the ZOE-50 study, 75.8 YOA in the ZOE-70 study, and 56.2 YOA in the ZOE-HSCT study. The percentage of men was 34.6%, 45.8%, and 53.5% in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively. The incidence rates of HZ were 9.1/1000 person years in both the ZOE-50 and ZOE-70 placebo groups and 95.6/1000 person years in the ZOE-HSCT study placebo group. The incidence was 84.9/1000 person-years and 99.3/1000 person-years in individuals aged 18–49 and ≥ 50 YOA in the ZOE-HSCT study, respectively. Supplementary Table S1 presents the incidence data for the ZOE-50 and ZOE-70 studies by age group, sex, and geographic region.
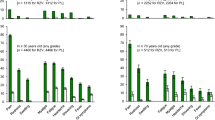
Table 2 presents the clinical assessment of the HZ cases for the three groups, respectively. More than 40% of participants in all groups reported prodromal pain, with the mean duration of prodromal pain varying from 2.9 days in the ZOE-HSCT study to 3.7 days in the ZOE-50 study, and 3.9 days in the ZOE-70 study. The majority of patients presented with a single dermatome affected. The percentages of patients with single adjacent dermatomes affected were 10.4% in the ZOE-50 study compared with 15.4% in the ZOE-70 study and 12.8% in the ZOE-HSCT study. The corresponding percentages with multiple dermatomes affected (i.e., beyond adjacent dermatome) were 10.0%, 10.4%, and 16.9%, respectively. In the ZOE-50 and ZOE-70 studies the most frequent location of the rash was on the upper trunk, whereas in the ZOE-HSCT study the most frequent location of rash was the lower trunk. The percentage of patients for whom rash was present in the head and eye locations appeared to be higher in the ZOE-70 study compared with the other studies. The percentage of patients for whom vesiculation was documented ranged from 84.3% in the ZOE-50 study through 88.8% in ZOE-70 to 93.0% in the ZOE-HSCT study.
Figure 1 presents the pain and rash locations. Pain was present in over 94.0% of patients in all three study groups (Table 2). Pain was rated as dull in approximately 36% of patients and was exacerbated by gentle touch (i.e., allodynia) in the majority of patients in all three study groups at the first clinical visit following HZ onset. The pain was described as constant for 42.1%, 32.5%, and 41.9% patients in the ZOE-50, ZOE-70, and ZOE-HSCT groups, respectively. More than 90% of patients were on medication to treat the HZ episode, with more than 80% on antiviral medication and more than 70% on pain medication (Table 3). Further details on the most frequently used medications are presented in Supplementary Table S2.
Table 4 presents the maximum worst pain scores during the HZ episode by study group, showing no major differences. In the three study groups, more than 60% of patients with HZ experienced severe pain, ≥ 86% experienced clinically relevant pain, and ≥ 92% experienced any pain. Table 5 presents the maximum interference of pain on ADL scores. The interference with ADLs tended to be highest in the ZOE-HSCT study. Sleep was the activity most impacted in the ZOE-50 and ZOE-70 study groups, whereas general activity was most impacted in the ZOE-HSCT study. The mean score for interference with normal work activities was 4.5 and 4.8 in the ZOE-50 and ZOE-70 studies, respectively, compared with 5.8 in the ZOE-HSCT study. Similarly, the mean score for interference with walking ability was 3.1 and 3.6 in the ZOE-50 and ZOE-70 studies, respectively, compared with 4.6 in the ZOE-HSCT study. Twenty patients in the ZOE-HSCT group were hospitalized because of HZ, compared with five in the ZOE-70 study and zero in the ZOE-50 study.
Table 6 and Fig. 2 present the duration of clinically significant pain. The median duration of clinically significant pain was estimated as 17 days (95% confidence interval (CI) 15–19 days) in the ZOE-50 study, 22 days (95% CI 18–27 days) in the ZOE-70 study, and 30 days (95% CI 22–36 days) in the ZOE-HSCT study. It was estimated that 12.3%, 16.9%, and 21.8% of patients in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively, had clinically significant pain persisting beyond 3 months (defined as PHN). Similarly, 10.5%, 9.9%, and 17.8% of patients in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively, had clinically significant pain persisting beyond 6 months. Censoring after loss to follow-up was a larger issue in the ZOE-HSCT study where 37.2% of participants were censored compared to 14.3% and 18.7% in the ZOE-50 and ZOE-70 studies, respectively (Table 6). Supplementary Table S3 shows that 3.2% and 8.3% participants in the ZOE-50 and ZOE-70 studies, respectively, continued to have clinically significant pain at their last ZBPI assessment compared with 12.2% of participants in the ZOE-HSCT study.
The AUC for the ZBPI worst pain score and the ZBPI ADL scores are presented in Supplementary Table S4. The mean AUC of the ZBPI worst pain score from the onset of HZ to day 182 post rash onset was 135.4, 189.7, and 173.3 in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively. Similarly, the mean AUC of the ZBPI interference with ADL score from the onset of HZ to day 182 post rash onset was 91.0, 132.7, and 124.0 in the ZOE-50, ZOE-70, and ZOE-HSCT studies, respectively.
Discussion
In this manuscript, we presented both the clinical and patient-reported outcome data from the placebo groups of the ZOE-50, ZOE-70, and ZOE-HSCT pivotal phase III clinical studies. A similar methodology for study design, case definition, data collection, and analysis was applied in all three studies allowing a comparison of data in immunocompetent and immunocompromised patients with HZ. There are very few prospective studies with robust sample sizes evaluating both clinical and patient-reported outcomes in immunocompromised individuals with HZ. The incidence of HZ was shown to be higher in the ZOE-HSCT study, consistent with previous data [20, 21]. Several publications have demonstrated that incidence rates of HZ tend to be higher among individuals who are immunocompromised or immunosuppressed as a result of disease or therapy, with incidence rates 1.5–3 times greater for individuals with autoimmune disease and up to seven-fold greater in patients with HSCT than those observed in age-adjusted healthy adults [21, 22].
Simultaneous involvement of multiple dermatomes and vesiculation of rash appeared to be more frequent in patients with HZ who were HSCT recipients [23]. Van Oorschot et al. reported that HZ causes difficulty with movement such as walking or doing physical activities, which is likely to be related to sensitivity of clothes touching the affected area and/or also pain that is exacerbated by movement [24]. These elements may explain the greater impact on walking ability and general activities observed in the HSCT group. The location of rash in the head and/or eye area appeared to be highest in older patients, predisposing to greater likelihood of ocular complications. Patients with HZ involving the first division of the trigeminal nerve typically present with ocular and periocular lesions [2]. Ocular complications (e.g., keratopathy, episcleritis, iritis, or stromal keratitis) may culminate in monocular blindness [25].
The majority of patients were on both antiviral and pain medication. Nonsteroidal anti-inflammatory drugs or acetaminophen or opioids are commonly used either as single agents or in combination for the treatment of acute pain and PHN associated with HZ [3]. The adverse effects of these medications can be additive, especially in elderly or immunocompromised patients who may also be on medication to treat underlying conditions [26].
Interestingly, the percentages of patients reporting any pain, clinically relevant pain, and severe pain were similar across the three study groups. Similarly, the AUC of the worst pain score during days 0–182 demonstrated a large disease burden for all three groups. The duration of clinically significant pain and proportion of patients with PHN was greatest in the HSCT study group. PHN is thought to be the result of virus- or immune response-induced damage to the affected sensory neurons and secondary changes in function at several levels of the nociceptive pathway [8]. Several authors reported that high proportions of HSCT recipients develop complications including PHN following HZ [20, 27, 28]. Another study demonstrated that individuals with an impaired immune status had HZ severity of illness scores, as measured by the ZBPI, that were twice as high as those of individuals with normal immune function [29].
Loss to follow-up was an issue, whereby some patients, in particular for the ZOE-HSCT study, discontinued completing ZBPI questionnaires while still experiencing either clinically relevant or severe pain at their last recording of pain (see Supplemental Table S3). Incomplete pain data has the consequence that unadjusted analysis (based on observed data only) such as the AUC analysis may result in the underestimation of the true pain observed by patients. Similarly, calculations of PHN based on observed data only would lead to underestimations of the proportion of subjects with PHN. As such, we performed the analysis of duration of clinically significant pain (see Fig. 2) using the Kaplan–Meier method which adjusts for missing data, consequently providing less biased estimates.
Although the risk and impact of HZ appears to be higher in all groups of severely immunocompromised populations, especially in those with autologous-HSCT, and currently only RZV has been demonstrated to be safe in these patients, the short-term immunogenicity of RZV in all these groups is good although lower than in immunocompetent patients. Similarly, HZ vaccines may have higher efficacy and longer duration of protection in immunocompetent populations. Further research is pending to evaluate the duration of protection against HZ after vaccination in various populations in order to inform health care practices. As such, it remains to be seen which populations will benefit most from vaccination in the long term.
Conclusion
The impact and complications of HZ are substantial, particularly in older adults and individuals who are immunodeficient or immunocompromised. More should be done to improve awareness of the disease and the benefits of prevention in high-risk patients. The current Advisory Committee on Immunization Practices recommendations and other recommending bodies reflect the favorable benefit/risk of RZV, where vaccination against HZ is recommended for immunocompetent adults aged 50+ years and in all adults who are or will be immunodeficient or immunosuppressed as a result of disease or therapy [30, 31].
References
Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8(1):37.
Gnann JW, Whitley RJ. Herpes zoster. N Engl J Med. 2002;347(5):340–6.
Cohen JI. Herpes zoster. N Engl J Med. 2013;369(3):255–63.
Volpi A, Gross G, Hercogova J, Johnson RW. Current management of herpes zoster. Am J Clin Dermatol. 2005;6(5):317–25.
Harpaz R, Ortega-Sanchez I, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(5):1–30.
Centers for Disease Control and Prevention. Prevention of zoster. MMWR Recomm Rep. 2008;57(RR-5):1–30.
Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6): e004833.
Johnson RW, Rice ASC. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–33.
Jung BF, Johnson RW, Griffin DRJ, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545.
Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: patient-reported outcomes in six European countries. J Public Health. 2012;20(4):441–51.
Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–56.
Gater A, Abetz-Webb L, Carroll S, Mannan A, Serpell M, Johnson R. Burden of herpes zoster in the UK: findings from the zoster quality of life (ZQOL) study. BMC Infect Dis. 2014;14(1):402.
Weinberg A, Levin MJ. VZV T cell-mediated immunity. Current topics in microbiology and immunology. Berlin: Springer; 2010. p. 341–57.
Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(1):S1–26.
Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96.
Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32.
Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33.
Curran D, Oostvogels L, Heineman T, et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol. 2019;74(8):1231–8.
Curran D, Matthews S, Rowley SD, et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT). Biol Blood Marrow Transpl. 2019;25(12):2474–81.
Yanni EA, Ferreira G, Guennec M, et al. Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the Clinical Practice Research Datalink 2000–2012. BMJ Open. 2018;8(6): e020528.
Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014;348: g2911.
Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328–37.
Wanders SLL, Miriam AM, Baak M. Risk factors for disseminated herpes zoster. Ned Tijdschr Geneeskd. 2020;164:D4825.
Van Oorschot D, McGirr A, Goulet P, et al. A cross-sectional concept elicitation study to understand the impact of herpes zoster on patients’ health-related quality of life. Infect Dis Therapy. 2022;11(1):501–16.
Davis AR, Sheppard J. Herpes zoster ophthalmicus review and prevention. Eye Contact Lens. 2019;45(5):286–91.
Lang PO, Martin Z-L, Pautex S. Herpes zoster and post-herpetic neuralgia in older adults. Rev Med Suisse. 2008;4(178):2398–404.
Offidani M, Corvatta L, Olivieri A, et al. A predictive model of varicella-zoster virus infection after autologous peripheral blood progenitor cell transplantation. Clin Infect Dis. 2001;32(10):1414–22.
Rogers JE, Cumpston A, Newton M, Craig M. Onset and complications of varicella zoster reactivation in the autologous hematopoietic cell transplant population. Transpl Infect Dis. 2011;13(5):480–4.
Drolet M, Brisson M, Levin MJ, et al. A prospective study of the herpes zoster severity of illness. Clin J Pain. 2010;26(8):656–66.
Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. Morb Mortal Wkly Rep. 2018;67(3):103–8.
Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). 2021. https://www.cdc.gov/vaccines/acip/recommendations.html. Accessed 10 Oct 2022.
Acknowledgements
Trademark
Shingrix is a trademark owned by or licensed to GSK.
Funding
GlaxoSmithKline Biologicals SA funded this study (110390, 113077) and took in charge all costs associated with the development and publication of this manuscript including the journal’s Rapid Service fees.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank the investigators of the ZOE studies for their support in the conception of the studies. The authors would also like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Desmond Curran, Kenneth Schmader, and Nicolas Lecrenier were involved in the conception and/or the design of the study. Anthony L Cunningham, Céline Boutry, Desmond Curran, Kenneth Schmader, and Nicolas Lecrenier participated in the collection/generation of the study data. All authors were involved in the interpretation of the data, reviewed and approved the final manuscript.
Prior Presentation
Data reported in this manuscript have been presented as a poster at ECCMID 2022, 23–26 April 2022 (Lisbon, Portugal).
Disclosures
Céline Boutry, Desmond Curran, and Nicolas Lecrenier are employed by/hold shares in GSK. Anthony L Cunningham received honoraria paid to his institution from GSK, Merck Serona (Merck), and BioCSL/Sequirus outside the submitted work. Sean Matthews reports personal fees from GSK during the conduct of the study and outside the submitted work. Anthony L Cunningham, Céline Boutry, Desmond Curran, Nicolas Lecrenier, and Sean Matthews declare no other financial and non-financial relationships and activities. Kenneth Schmader declares no financial and non-financial relationships and activities and no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
GSK makes available the anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to https://www.clinicalstudydatarequest.com. To request access to patient-level data and documents for this study, please submit an enquiry via https://www.clinicalstudydatarequest.com. Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Curran, D., Matthews, S., Boutry, C. et al. Natural History of Herpes Zoster in the Placebo Groups of Three Randomized Phase III Clinical Trials. Infect Dis Ther 11, 2265–2277 (2022). https://doi.org/10.1007/s40121-022-00689-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00689-7