Abstract
Introduction
Hereditary transthyretin (ATTRv, v for variant) amyloidosis is a rare, progressive, fatal disease with multisystem manifestations, caused by pathogenic variants in the transthyretin (TTR) gene. Vutrisiran, an RNA interference therapeutic that results in rapid TTR knockdown, improved neuropathy and quality of life (QOL) versus external placebo in patients with ATTRv amyloidosis with polyneuropathy in the phase 3 HELIOS-A study (NCT03759379). This post hoc analysis evaluates the impact of baseline neuropathy severity on response to vutrisiran treatment.
Methods
Patients were randomized (3:1) to vutrisiran (n = 122; 25 mg subcutaneous injection once every 3 months) or patisiran (n = 42; 0.3 mg/kg intravenous infusion once every 3 weeks), which served as a reference group. In this post hoc analysis, patients were grouped into quartiles of increasing baseline Neuropathy Impairment Score (NIS): Quartile (Q)1 ≥ 5.0 to ≤ 20.5; Q2 > 20.5 to ≤ 44.1; Q3 > 44.1 to ≤ 73.1; Q4 > 73.1 to ≤ 127.0. Mean change from baseline to Month 18 was summarized by quartile for a range of efficacy endpoints.
Results
Across all baseline NIS quartiles, vutrisiran demonstrated benefit versus external placebo in measures of neuropathy severity (modified NIS + 7), QOL (Norfolk Quality of Life-Diabetic Neuropathy), disability (Rasch-built Overall Disability Scale), gait speed (10-m walk test), and nutritional status (modified body mass index). Overall, patients in lower versus higher NIS quartiles (less severe neuropathy) at baseline maintained better scores at Month 18. The external placebo group progressively worsened in all measures at Month 18.
Conclusions
Vutrisiran demonstrated benefit in neurologic function and other key efficacy measures versus external placebo across all four baseline neuropathy severity quartiles. Patients initiating vutrisiran earlier in their disease course retained the highest neurologic function level after 18 months, highlighting the importance of early diagnosis and treatment.
Trial Registration Number
ClinicalTrials.gov: NCT03759379.
Similar content being viewed by others
Why carry out this study? |
Hereditary transthyretin (ATTRv, v for variant) amyloidosis, also known as hATTR amyloidosis, is a rare, progressive, and fatal disease, in which continued progression of neuropathy and/or cardiomyopathy leads to debilitating symptoms with increasing severity, impaired physical function, and decline in nutritional status and patients' quality of life (QOL). |
To better understand the disease trajectory of ATTRv amyloidosis, this post hoc analysis of the phase 3 HELIOS-A study assessed the response to vutrisiran treatment in measures of neuropathy, physical function, and QOL in patients with various baseline severities of neuropathy, as categorized by Neuropathy Impairment Score (NIS) quartiles. |
What was learned from the study? |
Vutrisiran provided treatment benefit compared with external placebo across a range of disease-relevant outcomes (neuropathy severity [modified NIS + 7], QOL [Norfolk Quality of Life–Diabetic Neuropathy], disability [Rasch-built Overall Disability Scale], gait speed [10-m walk test], and nutritional status [modified body mass index]) across all baseline NIS quartiles. |
Patients initiating vutrisiran earlier in their disease course (i.e., those in the lower NIS quartiles) retained the highest neurologic function level after 18 months. |
The findings of this study support the clinical benefit of vutrisiran as an effective treatment that can improve the lives of patients with ATTRv amyloidosis with polyneuropathy, regardless of baseline neuropathy severity, and highlight the importance of early diagnosis and treatment. |
Introduction
Hereditary transthyretin (ATTRv, v for variant) amyloidosis, also known as hATTR amyloidosis, is a rare, progressive, debilitating, and fatal disease caused by variants in the transthyretin (TTR) gene [1,2,3,4]. Pathogenic TTR variants lead to misfolding of TTR proteins, which accumulate as amyloid deposits in multiple organs and tissues [5, 6], including nerves, heart, gastrointestinal tract, and musculoskeletal tissues [1, 2, 4, 7]. As such, ATTRv amyloidosis is a multisystem disease with a heterogeneous clinical presentation typically involving sensory, motor, and autonomic neuropathy, and cardiomyopathy [2, 8,9,10], with the majority of patients presenting with a mixed phenotype of polyneuropathy and cardiomyopathy [11, 12]. ATTRv amyloidosis has an aggressive course, and disease progression is associated with increased symptom severity, decreased quality of life (QOL), loss of physical function, and death [3, 13, 14]. In untreated patients, prognosis is poor, with a median survival of 4.7 years following diagnosis [15], and a reduced survival of 3.4 years in patients with cardiomyopathy [16].
The natural course of ATTRv amyloidosis highlights the need for early and effective treatment that can minimize the burden of disease and the progressive worsening of QOL and physical function. The rapid progression of the disease when not treated is evident in natural history studies [3, 17, 18] and the placebo arms of pivotal clinical studies in patients with ATTRv amyloidosis [13, 19,20,21,22], in which outcomes measures related to neuropathy severity (e.g., modified Neuropathy Impairment Score + 7 [mNIS + 7]), QOL (Norfolk Quality of Life–Diabetic Neuropathy [Norfolk QOL-DN] questionnaire), disability (Rasch-built Overall Disability Scale [R-ODS]), and nutritional status (modified body mass index [mBMI]) steadily deteriorate over time.
Current disease-modifying treatment strategies for ATTRv amyloidosis include those that reduce levels of pathogenic TTR protein by silencing the TTR gene (RNA interference [RNAi] therapeutics; antisense oligonucleotides [ASO]), or those that stabilize the TTR tetramer (TTR stabilizers). These strategies have shown different levels of clinical benefit versus placebo in various manifestations in patients with ATTRv amyloidosis [19,20,21, 23].
The RNAi therapeutic patisiran, approved for treatment of patients with ATTRv amyloidosis with polyneuropathy [24], demonstrated the potential to halt polyneuropathy progression and improve multiple QOL and disability measures compared with placebo at 18 months in the pivotal phase 3 APOLLO study [13, 19]. A post hoc analysis also demonstrated that these improvements or stabilizations in neurologic function and QOL with patisiran versus placebo were evident across a wide range of baseline neuropathy severities [25]. Vutrisiran, another RNAi therapeutic that, like patisiran, acts by reducing the synthesis of both variant and wild-type TTR in the liver, and resulting in rapid knockdown of circulating TTR, has also been approved for the treatment of the polyneuropathy of ATTRv amyloidosis [26]. In the phase 3 HELIOS-A study in patients with ATTRv amyloidosis with polyneuropathy, vutrisiran met the primary endpoint of change from baseline in neuropathy impairment (mNIS + 7) compared with an external placebo group from the APOLLO study, as well as all secondary efficacy endpoints, and demonstrated an acceptable safety profile [27].
To better understand the disease trajectory of ATTRv amyloidosis, following a similar analysis to the aforementioned post hoc analysis of the APOLLO study, we report the efficacy of vutrisiran observed in the HELIOS-A study in patients with various baseline severities of neuropathy (as categorized by Neuropathy Impairment Score [NIS] quartiles).
Methods
Trial Design
The full methodology and study design details for the HELIOS-A study have been described previously [27]. In summary, the HELIOS-A study was a phase 3, global, randomized, open-label study of patients with ATTRv amyloidosis with polyneuropathy, and was conducted at 57 sites in 22 countries (NCT03759379). The study protocol and amendments were approved by relevant Institutional Review Boards or Independent Ethics Committees. Written informed consent was obtained from each participant. The study was conducted in accordance with all applicable regulatory requirements, the current guidelines of Good Clinical Practice, and principles originating from the Declaration of Helsinki.
Study Population
Eligible patients in the HELIOS-A study were aged 18–85 years with a documented TTR variant and diagnosis of ATTRv amyloidosis, neuropathy (baseline NIS of 5–130), a polyneuropathy disability score of ≤ IIIb, adequate liver and renal function, and a Karnofsky Performance Status (KPS) score of ≥ 60%. Patients who had received previous gene-silencing therapy, those with prior liver transplantation, those who were planned to undergo liver transplantation during the 18-month treatment period, and those with New York Heart Association class > II were excluded. Prior TTR stabilizer use was permitted, although patients were not allowed to use TTR stabilizers during their participation in the study.
Randomization and Treatment
Enrolled patients were randomized in a 3:1 ratio to 18 months of treatment with vutrisiran 25 mg subcutaneously once every 3 months or patisiran 0.3 mg/kg intravenously once every 3 weeks, which served as a reference group. The placebo group of the APOLLO study [19], which had similar eligibility criteria and endpoints to the HELIOS-A study, was used as an external placebo control for the primary endpoint and most secondary and exploratory endpoints.
Assessments
Full details of the primary, secondary, and exploratory efficacy and safety endpoints of the HELIOS-A study have been reported previously [27]. Briefly, the primary endpoint was the change in neuropathy impairment from baseline as measured by mNIS + 7 (range 0–304, with higher scores indicating greater neuropathy impairment) compared with the external placebo group of the APOLLO study at Month 9. mNIS + 7 was also assessed at Month 18 as a secondary endpoint. Here, we evaluate the impact of baseline neuropathy severity, as defined by baseline NIS quartiles, on the efficacy of vutrisiran in multiple outcome measurements such as neuropathy (mNIS + 7), QOL (Norfolk QOL-DN), functional status (R-ODS score), gait speed (10-m walk test [10-MWT]), and nutritional status (mBMI) over 18 months in patients treated with vutrisiran in the HELIOS-A study.
The NIS is a 244-point composite score derived from the assessment of muscle strength, reflexes, and sensation in the upper and lower limbs [28]. The mNIS + 7 score was developed from the NIS assessment and includes seven assessments in addition to the ones included in the NIS, which include five nerve conduction studies, a vibration detection threshold, and postural blood pressure [28]. For both NIS and mNIS + 7, a higher score indicates greater neurologic impairment. The 10-MWT assesses the time needed for a patient to walk 10 m, with longer time taken indicating worse ambulatory function. Norfolk QOL-DN is a 35-item questionnaire comprising five domains: physical functioning/large-fiber neuropathy, symptoms, activities of daily living, small-fiber neuropathy, and autonomic neuropathy (range – 4 to 136, with higher score indicating worse QOL) [29]. R-ODS is a 24-item, patient-reported scale that measures limitations in normal activities of daily living and social participation (range 0–48, with a lower score indicating more disability) [30]. Worse nutritional status is indicated by a lower mBMI score (weight/square of height [kg/m2] × serum albumin [g/L]). mBMI was chosen instead of BMI as patients with ATTRv amyloidosis may have low serum albumin levels due to malnutrition, leading to fluid retention and edema, which can result in increased weight and normal BMI measurements despite worsening nutritional status.
Statistical Analysis
Full details of the statistical analyses of the HELIOS-A study have been described previously [27]. For this post hoc subgroup analysis, patients from the vutrisiran group in the HELIOS-A study and the placebo group in the APOLLO study (external placebo) were divided into four quartiles based on increasing baseline NIS, from the least severe neuropathy impairment in quartile (Q)1: ≥ 5.0 to ≤ 20.5 (n = 50), then Q2: > 20.5 to ≤ 44.1 (n = 50) and Q3: > 44.1 to ≤ 73.1 (n = 50), to the most severe neuropathy impairment in Q4: > 73.1 to ≤ 127.0 (n = 49). Baseline demographics and disease characteristics were summarized by treatment group for each baseline NIS quartile. Data were descriptively summarized for the modified intent-to-treat population (defined as randomized patients who received any dose of study drug) as mean change from baseline at 9 and 18 months for each endpoint in each baseline NIS quartile. 10-MWT was calculated as the mean time (seconds) taken to complete two assessments at each visit, imputed as 0 for patients unable to perform the walk.
Results
Baseline Demographic and Disease Characteristics
Baseline characteristics by treatment group and by baseline NIS quartile are reported in Table 1. Baseline polyneuropathy, as assessed by NIS, ranged from 5 to 127 points, reflecting the range of neurologic impairment at baseline in both the HELIOS-A vutrisiran and the APOLLO placebo groups. In both groups, there were comparable proportions of patients in the respective Q2 and Q3 quartiles. In the vutrisiran group, there was a greater proportion of patients in Q1 at baseline, with the lowest proportion of patients in Q4. In contrast, in the external placebo group, the lowest and highest proportion of patients were in Q1 and Q4, respectively.
Median age was comparable between the external placebo and vutrisiran groups within each quartile, with patients in Q1 having a lower median age compared with patients in higher quartiles (Table 1). The patient populations in vutrisiran and external placebo groups were generally comparable within each quartile in terms of mNIS + 7, Norfolk QOL-DN, R-ODS, and mBMI. On the other hand, there were differences in baseline characteristics, including 10-MWT, KPS, percentage of patients with Val30Met TTR variant (V30M)/non-V30M, and percentage of patients meeting the definition of cardiac subpopulation between the treatment groups in some of the quartiles (Table 1).
For the external placebo group, there were treatment discontinuations in all NIS quartiles. Over time, a higher rate of discontinuation was observed in those patients with greater baseline neuropathy severity (3 of 12 patients [25.0%] in Q1 and 13 of 27 patients [48.1%] in Q4 discontinued treatment). For the vutrisiran group, there were no discontinuations in Q1 or Q4, while 2 of 32 (6.3%) patients in Q2 and 4 of 30 (13.3%) patients in Q3 discontinued treatment (Table 2). The reasons for treatment discontinuation across each treatment group and NIS quartile are listed in Table 2.
Polyneuropathy (mNIS + 7) Assessments by Baseline NIS Quartile
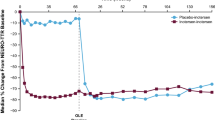
Within each baseline NIS quartile, baseline mNIS + 7 scores were generally comparable between the vutrisiran and external placebo groups (Table 1). Vutrisiran demonstrated a beneficial treatment effect on mNIS + 7 across all baseline NIS quartiles, relative to the external placebo group, which was first evident at Month 9 (Fig. 1).
When change in mNIS + 7 score from baseline was evaluated in different baseline NIS quartiles, vutrisiran-treated patients in Q1 and Q2 (i.e., less severe neuropathy at baseline) showed improved polyneuropathy at Months 9 and 18, as demonstrated by a negative mean change in mNIS + 7 score from baseline (Fig. 1). By Month 18, patients in Q3 and Q4 (i.e., more severe neuropathy at baseline) demonstrated modest worsening of polyneuropathy, as evidenced by relatively small increases in mNIS + 7 scores versus baseline. In contrast, across all baseline NIS quartiles, patients in the external placebo group demonstrated clear worsening of polyneuropathy at Months 9 and 18 versus baseline, with the mean change in mNIS + 7 scores ranging between 12.1 and 33.1. Although vutrisiran-treated patients experienced benefit compared with external placebo in all baseline NIS quartiles, patients in the higher quartiles were unable to achieve similar mNIS + 7 scores compared with those in the lower quartiles at Months 9 and 18.
Norfolk QOL-DN Assessments by Baseline NIS Quartile
Baseline Norfolk QOL-DN scores were generally comparable between patients in the vutrisiran and external placebo groups within each baseline NIS quartile (Table 1). Vutrisiran demonstrated a beneficial treatment effect on Norfolk QOL-DN across all baseline NIS quartiles relative to the external placebo group, which was first evident at Month 9 and continued through Month 18 (Fig. 2).
When change in neuropathy-related QOL from baseline was evaluated in different baseline NIS quartiles, vutrisiran-treated patients in Q1–Q3 showed improved QOL, as demonstrated by a negative mean change in Norfolk QOL-DN from baseline to 18 months (Fig. 2), and a relatively small deterioration of approximately 4.0 points was observed in patients in Q4 who had more severe neuropathy at baseline. In contrast, patients in the external placebo group experienced more prominent deterioration in their QOL for all baseline NIS quartiles; the mean change in Norfolk QOL-DN scores from baseline at Month 18 ranged between 10.9 and 25.6. Despite experiencing benefit relative to the external placebo group in all baseline NIS quartiles, vutrisiran-treated patients in the higher quartiles were unable to achieve the same level of QOL at 9 and 18 months compared with those in the lower quartiles.
Functional Measures (Gait Speed, R-ODS) and Nutritional Status (mBMI) Assessments by Baseline NIS Quartile
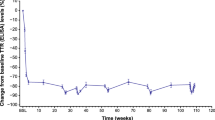
Baseline gait speed values in the vutrisiran and external placebo groups were comparable for Q3 and Q4, whereas, in the vutrisiran group, higher values were observed in Q1 and Q2 compared with the external placebo group (Table 1). In vutrisiran-treated patients, 10-MWT results remained stable in Q1–Q3 versus baseline, and patients in Q4 demonstrated a modest decline over 18 months (Fig. 3), whereas, in the external placebo group, there was a substantial decline in gait speed, which increased with each increasing quartile. Overall, vutrisiran treatment demonstrated a beneficial effect compared with external placebo across all baseline NIS quartiles, which was evident by 9 months for Q2–Q4.
Baseline R-ODS scores were generally comparable between the vutrisiran and the external placebo groups within each baseline NIS quartile (Table 1). For vutrisiran-treated patients, R-ODS scores remained relatively stable over 18 months across all baseline NIS quartiles, with a modest worsening in Q3 and Q4 versus baseline (Fig. 4). However, in the external placebo group, R-ODS showed clear worsening versus baseline over 18 months across all quartiles, with a larger decline observed with each increasing quartile. Vutrisiran showed a beneficial effect on R-ODS across all baseline NIS quartiles, relative to the external placebo group, which was first evident at Month 9.
For mBMI, baseline scores were comparable for the vutrisiran and the external placebo group within each baseline NIS quartile (Table 1). mBMI remained relatively stable over 18 months in vutrisiran-treated patients across all baseline NIS quartiles, with modest improvements from baseline in patients in Q1–Q3 and a small deterioration in patients in Q4 at 18 months (Fig. 5). Patients in the external placebo group experienced a relatively larger decline from baseline in mBMI across all baseline NIS quartiles. Thus, vutrisiran-treated patients experienced a treatment benefit on mBMI over the external placebo group across all baseline NIS quartiles over 18 months, which was first evident at Month 9.
Discussion
This post hoc analysis assessed the impact of baseline neuropathy severity on the outcomes of neurologic function, QOL, disability, and nutritional status in vutrisiran-treated patients with ATTRv amyloidosis with polyneuropathy from the HELIOS-A study compared with an external placebo group from the APOLLO study. Overall, vutrisiran demonstrated a beneficial treatment effect compared with external placebo for all endpoints at Month 18 across all subgroups of baseline neuropathy severity.
In the vutrisiran group, there was a trend for improvement or stabilization at Month 18 versus baseline across the different disease outcomes in patients from most baseline NIS quartiles. Although a modest amount of worsening was observed for some of the outcomes in patients who had more severe neuropathy at baseline (Q3 and/or Q4), vutrisiran continued to demonstrate a beneficial effect compared with external placebo even in those quartiles. In general, patients who had more severe neuropathy at baseline (Q3 and Q4) were not able to recover to the same level of function as those who initiated vutrisiran treatment earlier in their disease course (Q1 and Q2). These results demonstrate that earlier intervention allows greater opportunity to improve or stabilize disease-related endpoints, highlighting the benefits of early diagnosis and treating patients early in their disease course.
These observations also mirror those demonstrated in a similar post hoc analysis from the APOLLO study, where treatment benefit with another RNAi therapeutic, patisiran, versus placebo was demonstrated in patients with ATTRv amyloidosis across the full range of baseline NIS quartiles [25]. Previous studies evaluating the effect of TTR stabilizers on the NIS-Lower Limb or NIS assessments similarly found that patients with lower baseline disease severity were likely to have the greatest response to treatment, and thus the slowest disease progression [31,32,33]. Further, during the Italian compassionate use program for inotersen, patients with familial amyloid polyneuropathy (FAP) stage 1 at baseline demonstrated disease stability over 24 months, whereas those with FAP stage 2 at baseline showed worsening disease stage following treatment with inotersen [34]. Taken together, these data re-iterate the need for early and accurate diagnosis, and rapid treatment initiation in order to delay disease progression.
The availability of disease-modifying treatments, such as TTR gene silencers, has changed the treatment landscape of ATTRv amyloidosis [35]. In addition to these advanced treatment options, disease awareness has improved, leading to earlier diagnosis of patients [36,37,38], which—along with treating early to delay clinical progression and preserve QOL—is one of the key goals of disease management [35]. Our results are in line with these treatment goals and reinforce the benefits of vutrisiran, a disease-modifying treatment, across a wide range of baseline neuropathy severities.
Certain limitations when interpreting the data should be noted, including the post hoc nature of the analysis and its lack of power to report significant differences between the groups within each baseline NIS quartile due to the small sample sizes. Another limitation was the use of an external placebo control rather than a within-trial placebo group, although the APOLLO and HELIOS-A studies had similar eligibility criteria. Finally, the rate of study discontinuation in the HELIOS-A vutrisiran arm was low (4.1%) and similar to other studies conducted in the same patient population, and it was not considered to have an impact on the interpretation of the results of the current analysis.
Conclusions
Vutrisiran provides treatment benefits compared with external placebo across a range of disease-relevant outcomes regardless of baseline neuropathy severity. Furthermore, our data demonstrate the importance of early diagnosis and treatment to give patients the best opportunity to achieve stabilized or improved function and/or QOL in the progressive and debilitating disease process of ATTRv amyloidosis.
Data Availability
Anonymized individual participant data that support these results will be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and the EU. Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
References
Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11:50–7.
Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106:528–40.
Adams D, Coelho T, Obici L, et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85:675–82.
Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47:625–38.
Kelly JW. Amyloid fibril formation and protein misassembly: a structural quest for insights into amyloid and prion diseases. Structure. 1997;5:595–600.
Koike H, Katsuno M. Ultrastructure in transthyretin amyloidosis: from pathophysiology to therapeutic insights. Biomedicines. 2019;7:11.
Damy T, Judge DP, Kristen AV, Berthet K, Li H, Aarts J. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-Val30Met and non-Val122Ile hereditary transthyretin amyloidosis. J Cardiovasc Transl Res. 2015;8:117–27.
Shin SC, Robinson-Papp J. Amyloid neuropathies. Mt Sinai J Med. 2012;79:733–48.
Conceição I, Gonzalez-Duarte A, Obici L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21:5–9.
Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15:387–404.
Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–8.
Coelho T, Maurer MS, Suhr OB. THAOS—the Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76.
Obici L, Berk JL, González-Duarte A, et al. Quality of life outcomes in APOLLO, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. Amyloid. 2020;27:153–62.
Adams D, Algalarrondo V, Echaniz-Laguna A. Hereditary transthyretin amyloidosis in the era of RNA interference, antisense oligonucleotide & CRISPR-Cas9 treatments. Blood. 2023. https://doi.org/10.1182/blood.2023019884.
Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22:123–31.
Sattianayagam PT, Hahn AF, Whelan CJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33:1120–7.
Ines M, Coelho T, Conceicao I, Ferreira L, de Carvalho M, Costa J. Health-related quality of life in hereditary transthyretin amyloidosis polyneuropathy: a prospective, observational study. Orphanet J Rare Dis. 2020;15:67.
Wixner J, Mundayat R, Karayal ON, et al. THAOS: gastrointestinal manifestations of transthyretin amyloidosis—common complications of a rare disease. Orphanet J Rare Dis. 2014;9:61.
Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21.
Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31.
Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67.
González-Duarte A, Berk JL, Quan D, et al. Analysis of autonomic outcomes in APOLLO, a phase III trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. J Neurol. 2020;267:703–12.
Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92.
Alnylam Pharmaceuticals Inc. US prescribing information: ONPATTRO (patisiran) lipid complex injection, for intravenous use. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210922s000lbl.pdf. Accessed Nov 8, 2023.
Quan D, Obici L, Berk JL, et al. Impact of baseline polyneuropathy severity on patisiran treatment outcomes in the APOLLO trial. Amyloid. 2023;30:49–58.
Alnylam Pharmaceuticals Inc. US prescribing information: AMVUTTRA (vutrisiran) injection, for subcutaneous use: Food and Drug Administration. 2022. https://www.alnylam.com/sites/default/files/pdfs/amvuttra-us-prescribing-information.pdf. Accessed Nov 8, 2023.
Adams D, Tournev IL, Taylor MS, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30:1–9.
Dyck PJB, González-Duarte A, Obici L, et al. Development of measures of polyneuropathy impairment in hATTR amyloidosis: from NIS to mNIS+7. J Neurol Sci. 2019;405: 116424.
Vinik EJ, Vinik AI, Paulson JF, et al. Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2014;19:104–14.
van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76:337–45.
Gundapaneni BK, Sultan MB, Keohane DJ, Schwartz JH. Tafamidis delays neurological progression comparably across Val30Met and non-Val30Met genotypes in transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2017;25:464–8.
Amass L, Li H, Gundapaneni BK, Schwartz JH, Keohane DJ. Influence of baseline neurologic severity on disease progression and the associated disease-modifying effects of tafamidis in patients with transthyretin amyloid polyneuropathy. Orphanet J Rare Dis. 2018;13:225.
Monteiro C, Mesgazardeh JS, Anselmo J, et al. Predictive model of response to tafamidis in hereditary ATTR polyneuropathy. JCI Insight. 2019;4: e126526.
Luigetti M, Antonini G, Di Paolantonio A, et al. Real-life experience with inotersen in hereditary transthyretin amyloidosis with late-onset phenotype: Data from an early-access program in Italy. Eur J Neurol. 2022;29:2148–55.
Ando Y, Adams D, Benson MD, et al. Guidelines and new directions in the therapy and monitoring of ATTRv amyloidosis. Amyloid. 2022;29:143–55.
Adams D, Ando Y, Beirao JM, et al. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol. 2021;268:2109–22.
Ioannou A, Patel RK, Razvi Y, et al. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation. 2022;146:1657–70.
Kittleson MM, Ruberg FL, Ambardekar AV, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis. J Am Coll Cardiol. 2023;81:1076–126.
Acknowledgements
The authors would like to thank the patients and their families for their participation in the HELIOS-A study. The authors would like to thank the HELIOS-A study staff and members of the HELIOS-A Collaborators Group for their work on the study. A full list of the members of the HELIOS-A Collaborators Group is provided in the Supplementary Material.
Medical Writing, Editorial, and Other Assistance.
Medical writing assistance was provided by Kristen Brown, PhD from Adelphi Communications Ltd (Macclesfield, UK) in accordance with Good Publication Practice guidelines, and funded by Alnylam Pharmaceuticals.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was funded by Alnylam Pharmaceuticals Inc. The funder collaborated with authors during study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and took final responsibility for the decision to submit the manuscript for publication. Alnylam Pharmaceuticals Inc. funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Marco Luigetti, Dianna Quan, John L. Berk, Isabel Conceição, Yohei Misumi, Chi-Chao Chao, Shaun Bender, and David Adams all contributed to data interpretation, drafting, and critical review of the manuscript. Shaun Bender contributed to data analysis, data interpretation, drafting, and critical review of the manuscript. Emre Aldinc and John Vest contributed to study design/methodology, data interpretation, drafting, and critical review of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The HELIOS-A study (NCT03759379) was conducted in accordance with the International Conference of Harmonisation guidelines for Good Clinical Practice, local regulatory requirements, and the principles of the Declaration of Helsinki. The study was approved by Ethics Committees or Institutional Review Boards for each study site (listed in the Supplementary Material), and all patients provided written informed consent.
Conflict of Interest
Marco Luigetti reports consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Alnylam Pharmaceuticals, Pfizer, and Sobi. Dianna Quan reports research funding from Alnylam Pharmaceuticals, Avidity Biosciences, Cytokinetics, Ionis Pharmaceuticals, Janssen Pharmaceuticals (formerly Momenta Pharmaceuticals), Pfizer, and Viela Bio, and consulting fees from Alnylam Pharmaceuticals and Ionis Pharmaceuticals. John L. Berk reports research funding from Alnylam Pharmaceuticals, AstraZeneca, Eidos Therapeutics, and Ionis Pharmaceuticals, consulting fees from Alnylam Pharmaceuticals, AstraZeneca, Eidos Therapeutics, Intellia Therapeutics, and Ionis Pharmaceuticals, and data safety monitoring and/or advisory board membership for Alnylam Pharmaceuticals, AstraZeneca, Corino Therapeutics, Intellia Therapeutics, and Ionis Pharmaceuticals. Isabel Conceição reports consulting fees from Alnylam Pharmaceuticals, AstraZeneca, Pfizer, and Sobi, payment or honoraria for lectures, presentations, speakers bureaus, or educational events from Akcea Therapeutics, Alnylam Pharmaceuticals, AstraZeneca, and Pfizer, and data safety monitoring and/or advisory board membership for Akcea Therapeutics, Alnylam Pharmaceuticals, and Intellia Therapeutics. Yohei Misumi reports research funding and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Alnylam Pharmaceuticals and Pfizer. Chi-Chao Chao has nothing to disclose. Shaun Bender, Emre Aldinc, and John Vest are all employees of Alnylam Pharmaceuticals. Emre Aldinc and John Vest report ownership of equity in Alnylam Pharmaceuticals. David Adams reports consulting fees from Alnylam Pharmaceuticals and AstraZeneca.
Additional information
Prior Presentation: Some data from this manuscript have been presented at the Peripheral Nerve Society Annual Meeting, Copenhagen, Denmark, June 17–20, 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Luigetti, M., Quan, D., Berk, J.L. et al. Impact of Baseline Neuropathy Severity on Vutrisiran Treatment Response in the Phase 3 HELIOS-A Study. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00595-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00595-9