Abstract
The present study was undertaken to determine the effects of polyunsaturated fatty acids (PUFAs) on histomorphological structure of liver, developmental stages of ovary, serum calcium (Ca2+) level, sperm quality and spontaneous spawning performance of striped gourami, Colisa fasciatus. Fishes were collected from the Brahmaputra River, Mymensingh, Bangladesh. Treated group was fed with supplemental diet enriched with 1 % squid extracted phospholipids for 3 months while the control group was given the same feed except phospholipids. For histomorphology of liver and ovaries, samples were collected on monthly basis. Treated group exhibited higher gonadal maturation resulted in spontaneous spawning compared to control. During spawning time, normal morphological structure of hepatocytes with lipid granules was observed in the liver of treated group, whereas less lipid granules with altered histological appearance were noticed in control group. Moreover, serum Ca2+ concentration was measured significantly higher (P < 0.01) in PUFA-treated female in contrast with control one. Again, sperm quality was determined by staining the sperm with trypan blue dye. Live sperm depicted transparent appearance in contrast to the dead one that retained black spots. The amount of live sperm was significantly higher (98.86 ± 0.87 %) in PUFA-treated males than that of control (90.58 ± 0.67 %). Therefore, the present study clearly indicated these enhanced spawning performances of C. fasciatus owing to PUFAs supplementation in diet.
Similar content being viewed by others
Introduction
The striped gourami (Colisa fasciatus) belonging to the family Osphronemidae is one of the perch commonly found in Asia. It is in fact a dual purpose fish for its scrumptious taste, meeting the nutritional requirements of peoples as well as has ornamental values used in aquaria (Goodwin 2003). Many countries such as India, China, Germany, Hong Kong, Japan, Malaysia, Republic of Korea, Singapore, Taiwan, Thailand and USA are the main consumer of this fish (Tripathi 2004). The fish is omnivore in nature, therefore, it can easily be fed on live, frozen and flake feeds (Goodwin 2003). In the past, this species was readily available in freshwater pools, ditches, ponds, marshes, rivers and lakes with vegetation. The natural resources of this species are declining fast, especially in Bangladesh, due to drastic reduction of its natural feeding and breeding ground as a result of human intervention, climate change and habitats modification. At present C. fasciatus is under ‘lower risk-near threatened’ category although is not listed in the IUCN Red Data Book (http://www.redlist.org) and has become a high-priced fish (Rainboth 1996). It becomes sexually mature at 1 year of age while the total length of male and female reached about at 10 cm and 6–8 cm, respectively. The total life span of this species is around 4 years with three distinct life stages: pre-spawning, spawning and post-spawning period ranging from January to March, April to August and October to December, respectively (Mitra et al. 2007).
Many fish can be held in captivity and will spawn naturally when proper environment and appropriate diet are provided (Watanabe and Vassallo-Agius 2003). In teleosts, numerous nutritional-related factors such as feed ration, nutrient levels and compositions have been shown to influence various reproductive parameters such as gonadal development, gamete quantity and quality, spawning success, hatchability and larval quality (Izquierdo et al. 2001; Hachero-Cruzado et al. 2009). Polyunsaturated fatty acids (PUFAs) of the n-3 and n-6 series cannot be synthesized in vertebrates and must be provided through diet for the maintenance and regulation of cellular structure and function (Storebakken 2002). The fatty acid composition of the diet can also affect the overall reproductive performance in sea bass (Bruce et al. 1999) and gangetic leaffish (Reza et al. 2013). Dietary PUFAs, the precursors of eicosanoids such as prostaglandins (PGs), are involved in steroidogenesis and oocyte maturation in vertebrates (Sorbera et al. 1998). A great portion of these lipids are transferred to different parts of the body for expending various physiological processes such as migration and reproduction (Kandemir and Polat 2007). Liver performs important function of synthesizing vitellogenin (Vtg), a bulky and complex calcium (Ca2+)-binding phospholipo-glycoprotein (Patiño and Sullivan 2002) which appears in the blood of sexually maturing teleost fishes and other oviparous vertebrates (female) in response to circulating estrogen. Then, Vtg is taken up by the growing oocytes that transforms into egg yolk proteins (Arukwe and Goksøyr 2003). Increased level of Ca2+ concentration in the blood serum is an indicator of the augmentation of estrogen secretion (Tsai and Wang 2000) during ovarian maturation and reproductive cycle. Information is available on the biology of C. fasciatus particularly food habits, maturity, spawning and length weight relationships (Mitra et al. 2007), fecundity (Behra et al. 2005), effect of feed quality on growth and gonadal maturity (Chakrabarty et al. 2010), development of air breathing organ (Prasad et al. 1982), morpho-histology of the alimentary canal (Moitra and Ray 1977), sexual dimorphism (Dehadrai et al. 1973) and various physiological alterations during early life stages (Kumari and Prasad 1983) of C. fasciatus. However, the effects of PUFAs incorporation in diet for enhancing reproductive performances of C. fasciatus are yet quite limited. Therefore, the present study was undertaken to evaluate the effects of PUFAs on maturation enhancement of C. fasciatus that was recognized by the presence of ample lipid droplets in liver with early oocyte maturation that facilitated spontaneous spawning. We also examined the level of Ca2+ concentration in C. fasciatus serum attributable to the effects of marine squid extracted phospholipids.
Materials and methods
Collection of broodfish and rearing
Fishes were collected from the Brahmaputra River near the Shamvuganj Bridge, Mymensingh, Bangladesh. Upon arrival at the Mini Hatchery Complex, fishes were acclimatized, separated into male and female and kept in four cisterns (1.23 × 2.44 × 0.46 m) of which two cisterns were used for treatment and two for control. Each cistern has its individual inlet (provided with gentle shower) and outlet (covered by net) which allowed the renewal and removal of water continuously and there was no water recirculating system. Water level was maintained at 45 cm throughout the experimental period. The total body length and weight of female were recorded as 8.7 ± 1.2 cm and 17.41 ± 1.3 g, respectively, and that of male were recorded as 10.0 ± 1.3 cm and 19.25 ± 1.51 g, respectively. Then, 25 pairs of C. fasciatus were stocked in each cistern of control and treatment group while the male and female ratio was 1:1 and reared with the formulated diet for 3 months (February, March and April). Water hyacinth was placed in each cistern as suspended form and two thin wooden plates (1.8 × 0.35 m) at the bottom (positioned by means of bricks) to keep the water cool and provide shelter for the fishes. During the study, water temperature using a celsius thermometer, pH by a portable digital pH meter (MICRO-TEMP, pH 500) and dissolved oxygen (DO) by a digital DO meter (multi 340 i/set, DO-5509; Germany) were recorded as 25 ± 1 °C, 7.3 ± 0.1 and 6.5 ± 0.5 ppm, respectively.
Formulation of diet and feeding
Broodfish were given formulated diet prepared with the ingredients of fish meal (40 %), rice bran (20 %), wheat bran (15 %), maize meal (12.5 %), wheat flour (10 %), vitamin-B complex (0.5 %) (Techno Drugs, Narsingdi, Bangladesh), α-tocopherol (2.5 IU/g) (Square Pharmaceuticals Ltd., Bangladesh) and squid extracted phospholipids (1 %) (Nippon Chemical and Feed Co. Ltd., Hakodate, Hokkaido, Japan). The proximate compositions of the dietary ingredients were analyzed following the standard method given by Association of Official Analytical Chemists (AOAC (Association of Official Analytical Chemists) 1980). The diets were stored in refrigerator in air-tight plastic bag. The fishes were fed pellet diet twice daily, once in the early morning and next at afternoon (9:30 am and 5:30 pm) up to their satiety. However, only the treated group was fed PUFAs supplemental diet but control feeds were devoid of lipid. The composition of experimental feed is shown in Table 1.
Determination of fatty acid composition of squid meal phospholipids
Squid meal phospholipids were generous gifts from Nippon Chemical and Feed Co. Ltd. (Hakodate, Hokkaido, Japan). Squid meal phospholipids were examined in the Laboratory of Biomolecular Chemistry, Faculty of Fisheries, Hokkaido University, Hakodate, Japan for determination of fatty acid composition. Phospholipids were converted to methyl ester derivatives individually following the method of Prevot and Mordret (1976). The samples were dissolved in 1 ml n-hexane and 0.2 ml of methanolic 2 M NaOH solution was added. The mixture was shaken and kept at 50 °C for 20 s. Then 0.2 ml of methanolic 2 M HCl solution was added to it. The n-hexane layer was collected, concentrated and subjected to gas chromatographic analysis (Hitachi 163 gas chromatograph, Hitachi Co. Ltd., Ibaraki, Japan) using a 0.5 mm PEG-20 M liquid-phase-coated 40 m × 1.2 mm diameter G-300 column (Chemicals Evaluation and Research Institute, Saitama, Japan) and flame ionization detection. The temperatures of the column, detector and injector were 170, 250, and 240 °C, respectively. The identification of fatty acids was recognized by comparing the peak retention times with authentic standards (St. Louis, MO, USA) and following the method of Takahashi et al. (1988).
Determination of gonadosomatic index (GSI)
For the determination of GSI, two female fishes from each of PUFA-treated and control group were sacrificed separately during the months of February, March, April and May. Fishes and gonads were weighed by an electronic balance (METTLER TOLEDO, Switzerland). GSI was determined using the following formula:
GSI = (Gonad weight/Body weight) × 100 (Barber and Blake 2006).
Histomorphological study of liver and ovary of C. fasciatus
Samples (liver and ovary) for histomorphological observation were taken from the same fishes which were used for GSI estimation (as described above). Then the liver and ovary were preserved in 10 % neutral buffered formalin and Bouin’s fluid, respectively. Dehydration, cleaning and infiltration process were carried out in an automatic tissue processor (SHANDON, CITADEL 1000, England). Paraffin-embedded blocks were sliced by microtome machine (Leica Jung, RM 2035, Germany) at 5 µm size. The ribbons with samples were placed into a water bath (40 °C) and the best section was picked up by glass slides and placed on a slide drier (20 °C) for overnight. Then the slides were stained with haematoxylin and eosin dye. The stained sections were mounted with Canada balsam and photographed under a compound microscope (OPTIKA B-350, Italy).
Determination of serum Ca2+ level of C. fasciatus
Blood samples were collected by means of cardiac puncture with heparinized syringe from the same females that were used for estimation of GSI and histomorphological study. The serum was obtained after centrifugation at 15,000 rpm for 10 min and stored at −30 °C prior to analysis. The serum Ca2+ concentration was determined using the method of Blood Safety and Clinical Technology, World Health Organization (2006) (http://www.searo.who.int/en/Section10/Section17/Section53/Section481_1763.htm) with slight modification. 100 µl of serum was reacted with 2,000 µl of working reagent for in vitro quantitative estimation of Ca2+. Working reagent was prepared by mixing 25 mg O-cresolphthalein complexone (Sigma-aldrich, Germany) with 250 mg of coloring reagent 8-hydroxyquinoline (British Drug Houses, England). The solution was adjusted to 250 ml with distilled water and buffer (the buffer reagent was prepared using 7.56 g Tris-Hydroxymethyl-aminomethane dissolved in 50 ml distilled water, pH = 10.7). The entire equipments were carefully cleaned, rinsed with 3 % HCl and dried to remove traces of Ca2+. Total serum Ca2+ level was measured by a spectrophotometer at the absorbance level of 540 nm (SPECTRONICR GENESYS™5, USA). Serum Ca2+ concentrations were obtained using a standard curve of calcium carbonate and expressed as milligram per deciliter (mg/dl) of serum.
Determination of sperm quality
Sperm was taken from the testes by killing the male fishes. Sperm were released from the sperm mass into Ca2+-free saline and mixed-up well for homogeneity and counted by a hemacytometer. A 1 % stock solution of trypan blue dye (Nacalai Tesque, Inc., Kyoto, Japan) was prepared using Ca2+-free saline (pH = 7.4). A volume of 0.1 ml of the stock solution was poured to 0.9 ml of sperm suspension and diluted 100 times. Within 3–4 min, the number of live (unstained) and dead (stained) sperm was counted in ten random fields counting approximately 110 cells per field. The percentage of live sperm was calculated as:
Observation of spontaneous spawning and collection of fertilized egg
Ovulation and spermiation of fishes were ascertained by gently pressing the abdomen of few fishes periodically. After 3 months of intensive feeding trial, all the PUFA-treated groups were found ready for spawning in late April and the fish were kept under close observation. At that time C. fasciatus produced foam like bubbles occupied by fertilized eggs indicating spontaneous spawning in PUFA-treated cistern, whereas there were no such bubbles observed in control cisterns. Then, buoyant fertilized eggs with bubbles were collected.
Determination of fertilization and hatching
The fertilized eggs of C. fasciatus were transferred and spread in two trays (101.6 × 40.6 × 12.7 cm3) and two mini circular bowl hatcheries (50 L) with around 3,500 ± 50 eggs in each provided with gentle shower. Fertilization rate was determined with the help 0.2 ml scoops. After hatching, the numbers of hatchlings were carefully counted and the rate of hatching was estimated. Both fertilization rate and hatching rate were obtained from the formulae as given by Adebayo (2006).
Statistical analysis
Statistical analysis of sperm counting was performed using SPSS (SPSS, Chicago, IL, USA). The evaluation of the treatments was made by one-way analysis of variance (ANOVA) and Tukey’s post hoc test was conducted to determine specific differences in treatment means. The comparison of the serum Ca2+ concentration and GSI between PUFA-treated and controlled groups during different months were determined by paired samples T test using statistical software package SPSS (SPSS, Chicago, IL, USA). Differences were considered significant at an alpha of 0.05 (P < 0.05) and 0.01 (P < 0.01).
Results
Fatty acid composition in phospholipids and proximate analysis of ingredients
The percentage of palmitic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid, EPA and DHA in squid meal phospholipids is shown in Table 2. The result of analysis revealed that DHA was the highest amount (39.7 %) among the unsaturated fatty acids quantity followed by EPA (11 %). In case of saturated fats palmitic acid was measured highest amount (32.6 %). However, the other dietary ingredients (fish meal, rice bran, wheat bran, maize meal and wheat flour) contained some amount of lipids as well as other nutrients (Table 3) but their effects were balanced by supplementing the same amount to both study groups (treatment and control).
Histomorphological observation of liver and ovary of C. fasciatus
At the beginning of the experiment during the month of February, liver of the female C. fasciatus (Fig. 1a, b) demonstrated normal morphological structures indicated by absence of lipid deposition and condensed cytoplasm in the hepatocytes. However, in late April, prior to spawning, morphological changes in liver of treated (Fig. 1d) and control female (Fig. 1c) were compared. In treated group, vitellogenic female liver accompanied with the accumulation of lipid droplets and showed enlarged hepatocytes, increased nuclear diameter and large vacuoles in the cytoplasm (Fig. 1d), whereas the liver of control females had large amount of hepatocyte nuclear polarization, vacuolization in cytoplasm and isolated necrosis (Fig. 1c).
Histomorphological section of female C. fasciatus liver from control and PUFA-treated group at different months. a and b the liver of female C. fasciatus at the initial stage during the month of February, c liver of female C. fasciatus of control group at the month of late April, and d liver of vitellogenic female of PUFA-treated group at the month of late April. Hematoxylin and eosin staining at original magnification ×100. H hepatocytes, Nu nucleus, EH enlarged hepatocytes, LD lipid droplets, V vacuoles, N necrosis, PNu polarized nucleus
With regard to the ovary, the control group showed the cells at chromatin nucleolar stage (CN) and early perinucleolar stage (EP) in the month of February. The CN was identified by the small oocyte with scant cytoplasm, whereas EP was identified by the peripheral arrangement of a large number of small nucleoli (Fig. 2a). On the other hand, in the PUFA-treated ovarian cells, the EP and late perinucleolar stage (LP) were present simultaneously. The LP was identified by the oocyte of double in size to that of the EP stage (Fig. 2b). During the month of March, the control fish ovarian section demonstrated cortical alveoli (CA) stage as identified by the appearance of empty spherical vacuoles in the cytoplasm. The CA increased in number to form peripheral rows along with presence of LP stage (Fig. 2c). At the same time, treated group showed the CA, early yolk granule (EYG), and late yolk granule stage (LYG). The EYG was identified by the small and spherical yolk globule appeared in the peripheral region of cytoplasm and in LYG the yolk globules were increased in size and oil droplets appeared within the cytoplasm (vitellogenic stage) (Fig. 2d). Prior to spawning (late April) control ovary represented EYG, LYG, migratory nucleus (MN) and the pre-maturation stage (PM) stages. During MN stage the nucleus was progressively migrated towards the animal pole of the oocyte, yolk globules started to fuse together and being gradually bigger. The PM was detected by accumulation of hepatically derived yolk protein called Vtg responsible for majority of oocyte growth (Fig. 2e). Conversely, the treated ovary depicted the LYG, MN, PM, and the germinal vesicle breakdown (GVBD) stages. After GVBD the oocyte ovulated into the ovarian lumen and became mature or ripe eggs of gonadal development. During maturation, the yolk globules coalesced and nucleus was not observed. Afterward, the peripheral migration of the nucleus and the dissolution of its membrane were noted (Fig. 2f) and these were indicators of spawning period of treated group. The final stage of oocyte maturation was difficult to observe because of the shrinkage and distortion of these cells during normal tissue processing.
Photomicrograph of C. fasciatus ovary at different time courses. Controlled (a) and PUFA-treated (b) group at February, controlled (c) and PUFA-treated (d) group at March, controlled (e) and PUFA-treated (f) group at late April. CN chromatin nucleolar oocyte, EP early perinucleolar oocyte, LP late perinucleolar oocyte, CA cortical alveoli, EYG early yolk granules, GV germinal vesicle, OW oocyte wall, LYG late yolk granules (vitellogenic), PM pre-maturation oocyte, MN migratory nucleus of oocyte, RP ripe oocyte, LD lipid droplets (Hematoxylin and eosin staining at original magnification ×100)
Determination of serum Ca2+ level of C. fasciatus
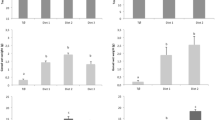
The effects of PUFAs on serum Ca2+ concentration (mg/dl) during different months are shown in Table 4. In both treated and control C. fasciatus female, Ca2+ level was found rising slowly at the month of February during early gonadal developmental stages. In treated female, just before spawning (late April) Ca2+ concentration was 3.699 ± 0.014 mg/dl, increased gradually for a certain period and after that it showed a sudden fall. Finally, the Ca2+ level was determined 1.171 ± 0.085 mg/dl during the month of May when the broods spawned spontaneously. A significantly higher Ca2+ level (mg/dl) was detected in PUFA-treated females than control ones during the month of March (P < 0.05), April, and May (P < 0.01).
Determination of fertilization and hatching
In late April, during the peak of the breeding season it was found that the quality of sperm of PUFA-treated C. fasciatus was better than that of control. The amount of live sperm was 98.86 ± 0.87 and 90.58 ± 0.67 % in the treated and control group, respectively. The percentage of live sperm-treated and control groups is shown in Fig. 3. Dead sperm retaining stain appeared as black spots whereas live sperm remained transparent without stain. More dark spots were found in control than treated group as illustrated in Fig. 4a, b. In late April, broods of C. fasciatus spawned spontaneously in the PUFA-treated cisterns but not in control cisterns. Fertilization and hatching rates were 93.45 ± 2.62 and 98.43 ± 3.03 %, respectively. Regarding GSI, significant difference (P < 0.01) was observed between two groups. During late April, PUFA-treated females showed the highest GSI of 4.3597 ± 0.5682 and during May, after spawning, the GSI was lowest 1.1870 ± 0.2104. Effects of PUFAs on GSI of female C. fasciatus on different months are shown in Table 5.
Discussion
The present results provide the evidence of vital role of dietary PUFAs on reproductive performances of C. fasciatus broodstock. In our study, the histomorphology of PUFA-treated C. fasciatus female liver showed numerous evenly distributed lipid droplets and normal morphological structures prior to spawning and that could be associated with a more favorable n-3 and n-6 fatty acid ratio in phospholipids. This result was supported by Robaina et al. (1998) who stated that supplementation of diet with n-3 PUFAs improves the utilization of liver lipids, thus reducing liver histomorphological alterations. Again, lipid droplets and condensed cytoplasm in enlarged hepatocytes were found in the liver of PUFA-treated matured female during the late April (prior to spawning). A similar study with Zoarces resulted active lipids deposition in liver occurs from March to August during the period of gonadal development (Pekkarinen and Kristoffersson 1975). These droplets must shift from the liver to the developing oocytes.
We observed that during early February, most of the eggs were at EP and LP stages in both treated and control group. After 1 month long feeding trial, that is, during the month of March, most of the eggs were found at CA, EYG and LYG stage in the treated fish ovary, whereas in the control fish ovary, eggs were found at yolk LP and CA stages. After 3 months of feeding trial (late April) the accumulation of the necessary yolk proteins completed in PUFA-treated ovary. This is understandable as we found the lipid globules at the vicinity of the nucleus which then moved to the oocyte periphery to cause oocyte maturation by hormonal stimulation. On the contrary, in case of control ovary, most of the eggs were still found at developmental stages (EYG, LYG, MN, and PM stages). This finding is in agreement with the result of Reza et al. (2013) who worked on effects of PUFAs on gonadal maturation in a perch fish Nandus nandus and found the similar strategies during the ovarian development in different months. The advanced oocyte developmental performance of treated female in contrast to the control is assumed to be due to the incorporation of PUFAs in the supplemental diet of treated group.
In the present experiment, the plasma Ca2+ levels and GSI of C. fasciatus, just prior to spawning (in late April), were found significantly higher (P < 0.05) in PUFA-treated female compared to the control and the values started to decrease after spawning (during May) in case of treated group. The results showed a positive correlations between serum Ca2+ concentration and GSI (r 2 = 0.9112 and r 2 = 0.8292 for treated and control group, respectively) which goes in line with the previous experiment where the existence of positive correlation between estrogens, plasma Ca2+ concentration and vitellogenesis in teleosts was noticed (Björnsson et al. 1989). Again, Nagler et al. (1987) and Hunn et al. (1992) also reported about linear relationship between increasing levels of serum Ca2+ and that of serum Vtg in case of female rainbow trout (Salmo gairdneri) and gravid female golden trout (Oncorhynchus aguabonita), respectively. Moreover, a steep decline in Ca2+ levels during the post-spawning phases was observed by Singh and Srivastav (1990) and Reza et al. (2013). Prior to vitellogenesis, increasing level of estrogens induces calcitonin secretion which in turn increases plasma Ca2+ level leading to Ca2+ binding by regulatory proteins like calmodulin, which turn the Ca2+ signal into a biological response (Clapham 2007). The difference between serum Ca2+ levels of treated female during different months corresponded to different types of sex hormone secretion resulting in enhanced egg production. The increase in blood plasma Ca2+ level depends on the high level of plasma estrogen secretion during sexual maturation (Lindhom 1997). In response to endogenous estrogen owing to dietary PUFAs via endocytic pathway, hepatocytes synthesize and release yolk proteins (Vtg) into the bloodstream, from where it was taken up and incorporated into growing oocytes (Shen et al. 1993). This appearance of Vtg in plasma ultimately increased the total plasma Ca2+ (Björnsson and Haux 1985). Therefore, the level of Ca2+ concentration in the blood serum was used as a biomarker for monitoring the gonadal maturation by introducing PUFAs in fish diet. The values of serum Ca2+ were comparatively lower in female C. fasciatus than in other fish species. Serum Ca2+ was measured 6.6–12.1 mg/dl for Acipenser brevirostrum (Knowles et al. 2006), and 7.21 mg/dl for A. fulvescens (Le Breton and Beamish 1998). These discrepancies might be due to the species differences, the blood-sampling procedure (caudal peduncle puncture versus cardiac puncture) and the influence of other blood parameters (for example, serum enzyme levels), or simply the differences between the fish collected from river, reservoir and beel or fish sizes. Another rationale may be the techniques were used to determine the serum Ca2+ levels that may vary. In the present investigation, male C. fasciatus was not taken into account as Singh and Srivastav (1990) claimed that there exist no correlation between serum Ca2+ level and testicular maturation.
In the present study, both n-3 (derived from 1 % phospholipids) and n-6 (in all dietary ingredients) fatty acids were included in the prepared diets. Rational amount of PUFA was added to the diet so that the C. fasciatus can synthesize their own EFA which is indispensable for enhancing their oocyte growth and maturity. We incorporated 1 % PUFAs with other dietary ingredients as treatment; after digestion, the dietary PUFAs were absorbed in intestine, transported to the blood and subsequently assimilated within tissues including brain, retina, heart, testes, ovaries and other tissues which enhanced both somatic and reproductive growth of C. fasciatus. In case of gilthead seabream and European sea bass, PUFA-enriched diet increased fecundity, fertilization and egg quality (Izquierdo et al. 2001). The preferential absorption of PUFAs followed by monoenes and saturated fatty acids had also been reported in tilapia (Ng et al. 2004). In the current study, we used PUFAs found from marine squid that might help increase the level of good cholesterol and reduce the low-density lipoproteins in fish liver. According to Hochberg (1998), cholesterol is synthesized by the liver and other body tissues through the steroidogenesis pathway generating major steroids that are involved in fish reproduction.
In this experiment, sperm quality, fertilization and hatching rate along with larval quality were found remarkably high in PUFA-treated group than control which resulted in physiological and morphological development of sperm as well as improvement of spawning performances of C. fasciatus. This result is supported by the previous experiment where important role of the PUFAs for proper spermatogenesis, sperm tail functioning and capacitation of sperm was explained (Reza et al. 2013). Furthermore, Yanes-Roca et al. (2009) stated that the use of fatty acid in diet has a significant positive correlations with fertilization percentage, hatching percentage and larval survival. The spontaneous spawning was observed in PUFA-treated C. fasciatus after three months feeding. Likewise, Reza et al. (2013) obtained natural spawning of Nandus nandus, feeding the brooders PUFAs supplemental diet.
The activities of PUFAs greatly depend on the n-3:n-6 ratio and vary from species to species, growth performances to spawning and it can be said that incorporation of n-3 fatty acids in reasonable amount is beneficial for fish as well as other animal health (Bruce et al. 1999). Based on our present finding we propose a conceptualized pathway showing the action of PUFAs on spontaneous spawning performance of C. fasciatus by a schematic representation as shown in Fig. 5. Dietary PUFAs are absorbed in small intestine and transported to blood and then taken up by the tissues. PUFA has direct influence on hypophysation and the PGsE2, an eicosanoid, and it stimulates steroid production. Besides, sensory stimuli originating externally by PUFAs are transduced by the brain so that the activities of specific neurohormones and neurotransmitters are appropriately modified for the secretion GnRH which in turn stimulates the pituitary gland for releasing gonadotropin (GtH I, GtH II). These hormones influence the thecal cell layer to secrete the androgenous substrate which diffuses into the granulosa cell layer where aromatase mediates for the final conversion of the androgens into the estrogens. The increased level of estrogens then induce calcitonin secretion that consequently increases plasma Ca2+ level, takes place prior to vitellogenesis. Increased Ca2+ concentrations lead to Ca2+ binding by regulatory proteins like calmodulin-mediating gonadotropin-induced ovarian steroidogenesis (as GnRH by hypothalamus) which turn the Ca2+ signal into a biological response. The Ca2+ levels can be used as indicators of plasma Vtg, alternative to monitor the gonadal maturation of C. fasciatus throughout different reproductive periods.
Proposed mechanistic pathway of PUFAs on reproductive performances of C. fasciatus. OA oleic acid, LA linoleic acid, AA arachidonic acid, NL neutral lipid, PE phosphatidal ethanolamine, PC phosphatidal choline, E2 estradiol-17β, PGE2 prostaglandin E2, Lv lipovitellin, Pv phosvitin, HDL high density lipoprotein, β′ β component, Zrp zonaradiata protein, LH luteinizing hormone, FSH follicle stimulating hormone, Δ-5, Δ-5 desaturase enzyme
In fine, such incorporation of PUFA as nutritional supplement to broodstock could lead to improve their reproductive performances. Further, if it is possible to make available these lipids to the farm owners for obtaining better maturation of their broods without using hazardous inducing agents, then the cost of farm operation will be decreased. The favorable outcome of the present study would highly be useful to repopulate and conserve the threatened fish in natural aquatic habitats. Thus, the risk of species extinction will be reduced that eventually improve the livelihood strategies of hatchery operators and fishermen.
References
Adebayo OT (2006) Reproductive performance of African Clariid Catfish Clarias gariepinus broodstock on varying maternal stress. J Fish Int 1(1–2):17–20
AOAC (Association of Official Analytical Chemists) (1980) In: Hoewitz W (ed) Official methods of analysis of association of official analytical chemists, 13th edn, Washington
Arukwe A, Goksøyr A (2003) Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol 2(4):1476–1486. doi:10.1186/1476-5926-2-4
Barber BJ, Blake NJ (2006) Reproductive physiology. In: Shumway SE, Parsons GJ (eds) Scallops: biology, ecology, and aquaculture, 2nd edn. Elsevier, Amsterdam, pp 357–406
Behra S, Khan MI, Das SK, Nagesh TS (2005) On the fecundity of stripped gourami, Colisa fasciata (Bloch and Schneider). J Inland Fish Soc India 37(1):68–70
Björnsson BT, Haux C (1985) Distribution of calcium, magnesium and inorganic phosphate in plasma of estradiol-17β treated rainbow trout. J Comp Physiol 155B:347–352
Björnsson BT, Haux C, Bern HA, Deftos LJ (1989) 17β-estradiol increases plasma calcitonin levels in salmonid fish. Endocrinology 125:1754–1760. doi:10.1210/endo-125-4-1754
Bruce M, Oyen F, Bell G, Asturiano JF, Farndale B, Ramos J, Bromage N, Carrillo M, Zanuy S (1999) Development of broodstock diets for the European sea bass (Dicentrarchus labrax) with special emphasis on the importance of n–3 and n–6 HUFA to reproductive performance. Aquaculture 177:85–97. doi:10.1016/S0044-8486(99)00071-X
Chakrabarty D, Das SK, Das MK (2010) Bag MP (2010) Low cost fish feed for aquarium fish: a test case using Colisa fasciata. Spanish J Agric Res 8(2):312–316
Clapham DE (2007) Calcium signaling. Cell 131(6):1047–1058. doi:10.1016/j.cell.2007.11.028
Dehadrai PV, Banerji SR, Thakur NK, Das NK (1973) Sexual dimorphism in certain air breathing teleosts. J Inland Fish Soc India 5:71–77
Goodwin D (2003) The practical aquarium fish handbook. Sterling, New York
Hachero-Cruzado I, Olmo P, Sánchez B, Herrera M, Domingues P (2009) The effects of an artificial and a natural diet on growth, survival and reproductive performance of wild caught and reared brill (Scophthalmus rhombus). Aquaculture 291:82–88
Hochberg RB (1998) Biological esterification of steroids. Endocr Rev 19(3):331–348. doi:10.1210/er.19.3.331
Hunn JB, Wledmeyer RJ, Greer IE, Grady AW (1992) Blood chemistry of laboratory-reared golden trout. J Aquatic Animal Health 4:218–222
Izquierdo MS, Fernández PH, Tacon AGJ (2001) Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197:25–42. doi:10.1016/S0044-8486(01)00581-6
Kandemir Ş, Polat N (2007) Seasonal variation of total lipid and total fatty acid in muscle and liver of rainbow trout (Oncorhynchus mykiss W., 1792) reared in Derbent Dam Lake. Turkish J Fish Aquatic Sci 7:27–31
Knowles S, Hrubec TC, Smith SA, Bakal RS (2006) Hematology and plasma chemistry reference intervals for cultured shortnose sturgeon (Acipenser brevirostrum). Vet Clin Pathol 35:434–440
Kumari P, Prasad MS (1983) Changes in the water-blood diffusion barrier of secondary gill lamellae during early life of Colisa fasciatus. Zool Anzeiger Jena 211:108–114
Le Breton GTO, Beamish FWH (1998) The influence of salinity on ionic concentrations and osmolarity of blood serum in lake sturgeon, Acipenser fulvescens. Environ Biol Fish 52:447–482
Lindhom S (1997) Sexual maturation in fish: studies of hepatic egg envelope proteins and vitellogenin during oogenesis. Candidatum Scientiarum Thesis, University of Bergen, Norway
Mitra K, Suresh VR, Vinci GK, Mazumdar NN, Biswas DK (2007) Biology and fishery of Banded Gourami, Colisa fasciata (Bloch and Schneider 1801) in a Floodplain Wetland of Ganga River Basin. Asian Fish Sci 20:409–423
Moitra SK, Ray AK (1977) Morpho-histology of the alimentary canal of an Indian fresh-water perch, Colisa fasciata (Bloch) in relation to food and feeding habits. Anat Anz 141(1):37–58
Nagler JJ, Ruby SM, Idler DR, So YP (1987) Serum phosphoprotein, phosphorus and calcium levels as reproductive indicators of vitellogenin in highly vitellogenic mature female and estradiol injected immature rainbow trout (Salmo gairdneri). Can J Zool 65:2421–2425
Ng WK, Sigholt T, Bell JG (2004) The influence of environmental temperature on the apparent nutrient and fatty acid digestibility in Atlantic salmon (Salmo salar L.) fed finishing diets containing different blends of fish oil, rapeseed oil and palm oil. Aquatic Res 35:1228–1237
Patiño R, Sullivan CV (2002) Ovarian follicle growth, maturation, and ovulation in teleost fish. J Fish Physiol Biochem 26:57–70
Pekkarinen M, Kristoffersson R (1975) Seasonal changes in concentrations of plasma lipids in the brackish-water eel-pout, Zoarces viviparus (L.). Ann Zool Fenn 12:260–262
Prasad MS, Mishra AP, Singh BR (1982) Development of the air breathing organ in Colisa fasciatus (Bl and Schn). Arch Ital Anat Embriol 87:243–261
Prevot AF, Mordret FX (1976) Utilisation des colonnes capillaries de verre pour l’analyse des corps gras par chromatographie en phase gazeuse. Rev Fse Corp Gras 23:409–423
Rainboth WJ (1996) FAO species identification field guide for fishery purposes. Fishes of the cambodian mekong. FAO, Rome
Reza AHMM, Rakhi SF, Hossen MS, Takahashi K, Hossain Z (2013) Enhancement of reproductive performances of Gangatic leaffish, Nandus nandus through up regulation of serum Ca2+ with the dietary polyunsaturated fatty acids. J Fish Physiol Biochem 39(4):779–791. doi:10.1007/s10695-012-9740-z
Robaina L, Izquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D (1998) Increase of the dietary n–3/n–6 fatty acid ratio and addition of phosphorus improves liver histological alterations induced by feeding diets containing soybean meal to gilthead seabream, Sparus aurata. Aquaculture 161:281–293. doi:10.1016/S0044-8486(97)00276-7
Shen X, Steyrer E, Retzek H, Sanders EJ, Chneider WJ (1993) Chicken oocyte growth: receptor-mediated yolk deposition. Cell Tissue Res 272:459–471
Singh S, Srivastav AK (1990) Changes in the serum calcium and phosphate levels in relation to the annual reproductive cycle of the freshwater catfish, Heteropneustes fossilis. Bull Physiol Animal 14:81–86
Sorbera LA, Zanuy S, Carrillo M (1998) A role for polyunsaturated fatty acids and prostaglandins in oocyte maturation in the sea bass (Dicentrarchus labrax). In: Vandry H, Tonon MC, Roubos EW, de Loof A (eds) Trends in comparative endocrinology and neurobiology: from molecular to integrative biology, vol 839. Annals of the New York Academy of Sciences, New York, pp 535–537
Storebakken T (2002) Atlantic salmon (Salmo salar). In: Webster CD, Lim CE (eds) Feeding of finfish for aquaculture. CAB International, Oxon, UK, pp 79–102
Takahashi K, Hirano T, Saito M (1988) Application of partition chromatographic theory for the analysis of marine triglyceride molecular species. Nippon Suisan Gakkaishi 54:523–528
Tripathi SD (2004) Ornamental fishes: breeding, culture and trade. In: Das RC, Sinha A, Datta S, Ghosh S (eds) Proceedings of the national seminar on prospects of ornamental fish breeding and culture in Eastern and North Eastern India. Central Institute of Fisheries Education, (Indian Council of Agricultural Research), Kolkata, India, pp 17–42
Tsai CL, Wang LH (2000) Sex differences in the responses of serum calcium concentrations to temperature and estrogen in tilapia, Oreochromis mossambicus. Zool Stud 39(1):55–60
Watanabe T, Vassallo-Agius R (2003) Broodstock nutrition research on marine finfish in Japan. Aquaculture 227:35–61
Yanes-Roca C, Rhody N, Nystrom M, Main KL (2009) Effects of fatty acid composition and spawning season patterns on egg quality and larval survival in common snook (Centropomus undecimalis). Aquaculture 287:335–340
Acknowledgments
The authors would like to extend immense gratitude to the authority of the Ministry of Science Technology (MOST), formerly, Ministry of Science and Information and Communication Technology (MOSICT), Government of the People’s Republic of Bangladesh, Dhaka, Bangladesh for providing financial help to carry out this piece of research work. The authors also acknowledge to Nippon Chemical and Feed Co. Ltd., Hakodate, Hokkaido, Japan for their generous gifts of squid meal phospholipids. The authors are ever grateful and immensely indebted to Mr. K. M. Golam Dastogeer, Department of Plant Pathology, Bangladesh Agricultural University, Mymensingh for his linguistic support during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hossen, M.S., Reza, A.H.M.M., Rakhi, S.F. et al. Effects of polyunsaturated fatty acids (PUFAs) on gonadal maturation and spawning of striped gourami, Colisa fasciatus . Int Aquat Res 6, 65 (2014). https://doi.org/10.1007/s40071-014-0065-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40071-014-0065-7