Abstract
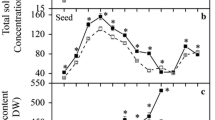
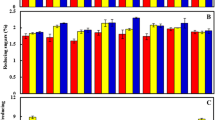
Isabgol is a commercially cultivated medicinal crop valued for its mucilaginous husk. Changes in sugar metabolism were studied in the developing seeds of Isabgol. Seed samples were collected after 7, 14, 21 and 28 days after anthesis (DAA). Results showed that the post anthesis reduction in soluble sugar content was 67 and 82.8 % in the seed and seed coat respectively. Starch and cellulose increased significantly in the seed after anthesis. Cell wall invertase activity increased after anthesis by 35.2 % and was the highest at 21 DAA. Acid invertase activity did not change significantly during seed development. Sucrose synthase (SuSy) activity increased from 0.12 to 0.34 µmol min−1 mg protein−1 till 28 DAA. Sucrose phosphate synthase (SPS) activity was the highest at 21 DAA (3.81 µmol min−1 mg protein−1). ADP glucose pyrophosphorylase (ADPGlc-PPase) activity also increased post anthesis and increase was four times till 21 DAA and enzyme activity was 12.78 µmol min−1 mg protein−1 at 21 DAA. Present study revealed that cell wall invertase and SuSy are the main enzymes involved in sugar breakdown during seed development in isabgol, while SPS and ADPGlc-PPase were important during storage phase.




Similar content being viewed by others
References
Mehta DM, Shelat PK, Parejia PB, Patel AJ, Barot B (2011) Investigations of Plantago ovata husk powder as a disintegrating agent for development of famotidine tablets. Int J Pharm Sci Nanotechnol 4(2):1412–1417
Kennedy JF, Sandhu JS, Southgate DAT (1979) Structural data for the carbohydrate of ispaghula husk ex Plantago ovata forsk. Carbohydr Res 75:265–274
Yang X, Baskinc JM, Baskinc CC, Huanga Z (2012) More than just a coating: ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect Plant Ecol 14:434–442
Zhou L, Paull RE (2001) Sucrose metabolism during papaya (Carica papaya) fruit growth and ripening. Am Soc Hortic Sci 126(3):351–357
Sturm A (1999) Invertases primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Leigh RA, Rees TA, Fuller WA, Banfield J (1979) The location of acid invertase activity and sucrose in the vacuoles of storage roots of beetroot (Beta vulgaris). Biochem J 178:539–547
Iwatsubo T, Nakagawa H, Ogura N, Hirabyashi T, Sato T (1992) Acid invertase of melon fruits: immunochemical detection of acid invertases. Plant Cell Physiol 33:1127–1133
Kleczkowski LA, Kunz S, Wilczynska M (2010) Mechanisms of UDP-glucose synthesis in plants. Crit Rev Plant Sci 29:191–203
Ruan YL, Llewellyn DJ, Liu Q, Xu SM, Wu LM, Wang L, Furbank RT (2008) Expression of sucrose synthase in the developing endosperm is essential for early seed development in cotton. Funct Plant Biol 35:382–393
Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189:329–339
Huber SC, Huber JL (1992) Role of sucrose-phosphate synthase in sucrose metabolism in leaves. Plant Physiol 99:1275–1278
Smith AM, Denyer K, Martin C (1997) The synthesis of starch granule. Annu Rev Plant Phys 48:67–87
Jeng TL, Tsenga TH, Wanga CS, Chenb CL, Sungc JM (2003) Starch biosynthesizing enzymes in developing grains of rice cultivar Tainung 67 and its sodium azide-induced rice mutant. Field Crop Res 84:261–269
Vigeolas H, Mohlmann T, Geigenber P (2004) Embryo specific reduction of ADP-Glc pyrophorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant Physiol 136(1):2676–2686
Fallahi H, Scofield G, Badger MR, Chow WS, Furbank RT, Ruan YL (2008) Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J Exp Bot 59:3283–3295
Aquila MEA, Braga MR, Dietrich SMC (2012) The similarity of galactomannan in seeds and endocarp of pods during development in Senna macranthera var. nervosa. S Afr J Bot 83:56–62
Hyde BB (1970) Mucilage producing cells in the seed coat of Plantago ovata: developmental fine structure. Am J Bot 57(10):1197–1206
Dubios M, Gilles KA, Hamilton JK, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Annu Rev Plant Phys 32:420–424
Weber H, Borisjuk L, Heim U, Buchner P, Wobus U (1995) Seed coat-associated invertases of faba bean control both unloading and storage functions: cloning of cDNAs and cell type-specific expression. Plant Cell 7:1835–1846
Xu DP, Sung SJS, Loboda T, Kormanik PP, Black CC (1989) Characterization of sucrolysis via the uridine phosphate and pyrophosphate-dependent sucrose synthase pathway. Plant Physiol 90:635–642
Stitt M, Wilke I, Feil R, Heldt HW (1988) Coarse control of sucrose-phosphate synthase in leaves: alterations of the kinetic properties in response to the rate of photosynthesis and the accumulation of sucrose. Planta 174:217–230
Smith AM (1990) Enzymes of starch synthesis. Meth Plant Biochem 3:93–102
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Babb VM, Haigler CH (2001) Sucrose phosphate synthase activity rises in correlation with high-rate cellulose synthesis in three heterotrophic systems. Plant Physiol 127:1234–1242
Ruan YL, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase expression represses cotton fibre cell initiation, elongation and seed development. Plant Cell 15:952–964
Acknowledgments
The author acknowledges the Director, Directorate of Medicinal and Aromatic Plants Research, for providing the necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bansal, R. Cell Wall Invertase and Sucrose Synthase Regulate Sugar Metabolism During Seed Development in Isabgol (Plantago ovata Forsk.). Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 73–78 (2018). https://doi.org/10.1007/s40011-016-0736-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0736-9