Abstract
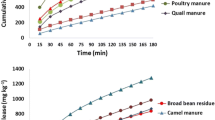
To evaluate different mathematical models for describing cumulative release of potassium (K) and silicon (Si) from soil by citric acid, an experiment was carried out in packed columns with three contrasting soils of India representing Alfisols, Vertisols and Inceptisols. The soils were saturated with six concentrations of citric acid viz. 0, 5, 10, 20, 40, 100 mg L−1 prepared in 0.01 M CaCl2 solution and the soil solution was collected by liquid displacement method at 15, 30, 45, 60 day intervals and analyzed for K and Si. These data were fitted to mathematical models viz. linear, quadratic, exponential, logarithmic, and power to describe such release. The results revealed that largest release of K and Si was observed in the 100 and 40 mg L−1 citric acid treatment, respectively. In general, the relationship between citric acid concentration and cumulative release of K and Si fitted well to quadratic equations (y = ax 2 + bx + c). Release of K with time was fitted the best in power form (y = at b), while the release of Si was fitted the best in linear equation (y = mx + c). These empirical equations may help in understanding the behavior of the soils towards application of citric acid. Also, information deduced from mathematical models could help in explaining the release mechanisms and to estimate the potassium and silicon supplying power of soils due to the action of citric acid.



Similar content being viewed by others
References
Uren NC (2000) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The Rhizosphere: Biochemistry and organic substances at the soil-plant interface. Marcel Dekker Inc, New York, pp 19–40
Chiang KY, Wang YN, Wang MK, Chiang PN (2006) Low-molecular-weight organic acids and metal speciation in rhizosphere and bulk soils of a temperate rain forest in Chitou, Taiwan. Taiwan J For Sci 21:327–337
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139
Van Hees PAW, Lundström US, Giesler R (2000) Low molecular weight acids and their Al complexes in soil solution-composition, distribution and seasonal variation in 3 podzolized soils. Geoderma 94:173–200
Mccoll JG, Pohlman AA (1986) Soluble organics and their chelating influence on aluminium and other metal dissolution in forest soils. Water Air Soil Pollut 31:917–927
Chatterjee D, Datta SC, Manjaiah KM (2015) Effect of citric acid treatment on release of phosphorus, aluminium and iron from three dissimilar soils of India. Archives Agron Soil Sci 61:105–117. doi: 10.1080/03650340.2014.919449
Datta SC, Takkar PN, Verma UK (2009) Effect of partial removal of adsorbed humus on kinetics of K and silica release by tartaric acid from clay–humus complex from 2 dissimilar soil profiles. Aust J Soil Res 47:715–724
Chatterjee D, Datta SC, Manjaiah KM (2014) Transformation of short-range order minerals in maize (Zea mays L.) rhizosphere. Plant Soil Environ 60:241–248
Violante A, Huang PM (1985) Influence of inorganic and organic ligands on the formation of aluminium hydroxides and oxyhydroxides. Clays Clay Miner 33:181–192
Piper CS (1950) Soil and Plant Analysis. Hans Publishers, Mumbai, p 368
Walkley A, Black IA (1934) An examination of degtjareff method for determining soil organic and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Subbiah BV, Asija GL (1956) A rapid procedure for assessment of available nitrogen in soils. Curr Sci 25:259–260
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ p 939. Washington DC, USA
Bray RH, Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–42
Murphy J, Riley JP (1962) A modified single method for determination of phosphates in natural waters. Anal Chem Acta 27:31–36
Hanway JJ, Hiedal H (1952) Soil analysis method used in Iowa State Soil Testing Laboratory. Iowa Agric. 57, 1–31. In: Black CA. (ed) Methods of Soil Analysis, part 2, American Society of Agronomy, Medison, pp 1025–1027
Wood LK, De Turk EE (1941) The adsorption of potassium in soils in non-replaceable form. Soil Sci Soc Am Proc 5:152–161
Bouyoucos GT (1951) A recalibration of hydrometer for mechanical analysis of soil part 1. Agron J 43:234–253
Jackson ML (1975) Soil Chemical Analysis—Advanced Course. Published by the author. Department of soils. University of Wisconsin, Madison
Kumar M, Ghosh SK, Manjaiah KM (2002) Components of naturally occurring organo-mineral complexes in some Inceptisols of India. Clay Res 21:59–74
Weaver RM, Syres JK, Jackson ML (1968) Determination of silica in citrate-bicarbonate-dithionite extracts of soils. Soil Sci Soc Am Proc 32:497–501
Jardine PM, Sparks DL (1984) Potassium–calcium exchange in a multireactive soil system: I. Kinetics. Soil Sci Soc Am J 48:39–45
Panse VG, Sukhatme PV (1962) Statistical methods for agricultural workers, 2nd edn. I.C.A.R, New Delhi
Snedecor GW, Cochran WG (1967) Statistical methods, 5th edn. Oxford and IBH. Pub. Co. Eton Press, Calcutta
Robert M (1973) The experimental transformation of mica toward smectite; relative importance of total charge and tetrahedral substitution. Clays Clay Miner 21:167–174
Srinivasarao Ch, Rupa TR, SubbaRao A, Ramesh G, Bansal SK (2006) Release kinetics of nonexchangeable K by different extractants from soils of varying mineralogy and depth. Comm Soil Sci Plant Anal 37:473–491
Sauer D, Saccone L, Conley DJ, Herrmann L, Sommer M (2006) Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 80:89–108
Cheng T, Hammond DE, Berelson WL, Hering JG, Dixit S (2009) Dissolution kinetics of biogenic silica collected from water column and sediments of 3 Southern California borderland basins. Marine Chem 113:41–49
Pansu M, Gautheyrou J (2006) Mineralogical separation by selective dissolution. Handbook of soil analysis. Mineralogical, organic and inorganic methods. Springer, New York, pp 167–219
Jat RS, Meena HN (2014) Pod yield and phosphorus-use efficiency in groundnut (Arachis hypogaea) as influenced by citric acid and its delivery methods under a semi-arid agro-ecosystem. Indian J Agron 59:106–111
Acknowledgments
The authors thank the Head, Division of Soil Science and Agricultural Chemistry, Indian Agricultural Research Institute, New Delhi, India, for providing all the required facilities to carry out the present investigation. None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, D., Datta, S.C. & Manjaiah, K.M. Citric Acid Induced Potassium and Silicon Release in Alfisols, Vertisols and Inceptisols of India. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 86, 429–439 (2016). https://doi.org/10.1007/s40011-014-0464-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-014-0464-y