Abstract
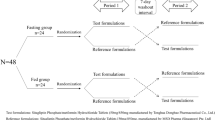
The objective of current study was to develop metformin hydrochloride modified release small sized tablets, and to evaluate its bioequivalence when compared with the reference product (Glucophage® SR). The physicochemical properties of the formulated and reference tablets were determined and compared. The in vitro dissolution profiles of formulated tablets were obtained using bio-relevant media and IVIVC was established. Bioequivalence investigation was carried out in 32 healthy male volunteers who received a single dose 1,000 mg of both test and reference products in a randomized two-way crossover design in postprandial conditions. After dosing, serial blood samples were collected for a period of 48 h. Metformin concentration was assayed by using a validated LC–MS/MS method. The log-transformed Cmax and AUC were statistically compared by analysis of variance, and the 90 % confidence intervals (CI) of the ratio of the log-transformed Cmax and AUC between the most promising developed formulation and the reference product were determined. It was deduced that the dissolution rate profile of the formulated modified release small sized tablets was similar to the reference product employed in the study. Their similarity and difference factors were found to be well within the established limits. In the bioequivalence study, the difference in Cmax, AUClast and AUCinf between the test and reference product was not statistically significant, with the 90 % CI of 100.73–116.20, 86.16–97.37 and 87.12–96.99 %, respectively. The results suggested that the metformin small tablets formulated with reduced quantity of controlled release polymer were found to be bioequivalent in the postprandial state. For these small tablets, pH 5.5 acetate buffer as a bio-relevant dissolution media for the development of IVIVC.










Similar content being viewed by others
References
Balan G, Timmins P, Greene D, Marathe P (2001) In vitro–in vivo correlation (IVIVC) models for metformin after administration of modified-release (MR) oral dosage forms to healthy human volunteers. J Pharm Sci 90:1176–1185
Bretnall A, Clarke G, Harry G (1998) Metformin hydrochloride. Analytical profiles of drug substances and excipients. Academic Press, San Diego
Channer K, Virjee JP (1986) The effect of size and shape of tablets on their esophageal transit. J Clin Pharmacol 26:141–146
Cheng C, Yu L, Lee H, Yang C, Lue C, Chou C (2004) Biowaiver extension potential to BCS Class III high solubility-low permeability drugs: bridging evidence for metformin immediate-release tablet. Eur J Pharm Sci 22:297–304
Chou C (2000) Uptake and dispersion of metformin in the isolated perfused rat liver. J Pharm Pharmacol 52:1011–1016
Costa P, Sousa Lobo J (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–133
Cutler D (1981) Assessment of rate and extent of drug absorption. Pharmacol Ther 14:123–160
Graham G, Punt J, Arora M et al (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98
Guidance (2013) Size, shape, and other physical attributes of generic tablets and capsules (draft). Accessed on 13 Dec 2013. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM377938.pdf
Higuchi T (1963) Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149
Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
Nicklin P, Keates A, Page T, Bailey C (1996) Transfer of metformin across monolayers of human intestinal Caco-2 cells and across rat intestine. Int J Pharm 128:155–162
Pentikainen PJ, Neuvonen PJ, Penttila A (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 16:195–202
Peppas NA (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110–111
Scheen A (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30:359–371
Vidon N, Chaussade S, Noel M, Franchisseur C, Huchet B, Bernier J (1988) Metformin in the digestive tract. Diabetes Res Clin Pract 4:223–229
Wamberg T, Jorgensen F, Hasselbalch H, Hey H (1983) The prejudgement of the esophageal transfer of tablets and capsules. Arch Pharm 11:24–31
Acknowledgments
Authors would like to thank Dr. Reddy’s Laboratories for providing assistance and support to publish this work.
Conflict of interest
All the authors are employees of Dr. Reddy’s laboratories and have no competing interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eaga, C., Mantri, S., Malayandi, R. et al. Establishing postprandial bio-equivalency and IVIVC for generic metformin sustained release small sized tablets. Journal of Pharmaceutical Investigation 44, 197–204 (2014). https://doi.org/10.1007/s40005-013-0115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-013-0115-y