Abstract
Background
Tocilizumab and baricitinib are recommended treatment options for hospitalized COVID-19 patients requiring oxygen support. Literature about its efficacy and safety in a head-to-head comparison is scarce.
Methods
Hospitalized COVID-19 patients requiring oxygen were treated with tocilizumab or baricitinib additionally to dexamethasone. Tocilizumab was available from February till the 19th of September 2021 and baricitinib from 21st of September. The primary outcome was in-hospital mortality. Secondary outcome parameters were progression to mechanical ventilation (MV), length-of-stay (LOS) and potential side effects.
Results
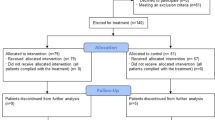
159 patients (tocilizumab 68, baricitinib 91) with a mean age of 60.5 years, 64% male were included in the study. Tocilizumab patients were admitted 1 day earlier, were in a higher WHO category at the time of inclusion and had a higher CRP level on admission and treatment initiation. Patients receiving Tocilizumab were treated with remdesivir more often and only patients in the baricitinib group were treated with monoclonal antibodies. Other characteristics did not differ significantly. In-hospital mortality (18% vs. 11%, p = 0.229), progression to MV (19% vs. 11%, p = 0.173) and LOS (13 vs. 12 days, p = 0.114) did not differ between groups. Side effects were equally distributed between groups, except ALAT elevation which was significantly more often observed in the tocilizumab group (43% vs. 25%, p = 0.021).
Conclusions
In-hospital mortality, progression to MV and LOS were not significantly different in patients treated with tocilizumab or baricitinib additionally to standard of care. Both drugs seem equally effective but further head-to-head trials are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
COVID-19 is caused by the SARS-CoV-2 and manifest with a wide variety of symptoms as well as severity. Most patients have a- or oligosymptomatic infections with predominantly upper respiratory symptoms. Especially patients with risk factors may develop moderate to severe COVID-19 with the need for hospitalization and oxygen support. In a small number of patients the disease progresses to a critical and life-threatening illness with the need for treatment in intensive care units (ICU) and mechanical ventilation [1,2,3,4,5].
The optimal treatment of COVID-19 is complex and depends on the stage of the disease [1, 2]. At early stages where viral replication is predominant antiviral treatment should be initiated. The two oral antivirals molnupiravir and nirmatrelvir/ritonavir have shown to reduce the combined endpoint hospitalization and death at day 29 significantly in outpatients at risk for severe disease when administered within 5 days of symptom onset [6, 7]. The first available antiviral remdesivir which must be administered intravenously has shown to reduce the need for hospitalization in outpatients [8], progression to mechanical ventilation and in-hospital mortality in various observational [9,10,11,12] and randomized controlled trials (RCTs) [13,14,15,16]. Remdesivir seems to be most beneficial in patients with need for oxygen but not on mechanical ventilation [13, 16].
In advanced disease the inflammatory response of the host can result in a cytokine storm which leads to clinical deterioration [17,18,19]. Several anti-inflammatory drugs were shown to be beneficial. Dexamethasone reduced mortality in COVID-19 patients with need for oxygen support (number needed to treat, NNT = 25) with an even more pronounced effect in patients who were mechanically ventilated (NNT = 8) [20]. The interleukin-6 antagonist tocilizumab reduced progression to MV (NNT = 13) and mortality (NNT = 20) additionally to corticosteroids in a metanalysis of 19 RCTs [21].
The JAK inhibitor baricitinib reduced the secondary endpoints all-cause and 60-day mortality (both NNT = 20) but failed to reduce the combined primary endpoint progression to high-flow oxygen, non-invasive ventilation, invasive ventilation or death in the COV-BARRIER RCT in patients with COVID-19 and need for oxygen [22]. Tofacitinib another JAK inhibitor reduced the primary endpoint progression to death or respiratory failure through day 28 (NNT = 9) and the secondary endpoint death through day 28 (NNT = 37) in a small RCT [23]. Approximately 90% of the patients received corticosteroids in both trials [22, 23]. These results support the use of tocilizumab and JAK inhibitors additionally to corticosteroids in patients with severe COVID-19.
Both tocilizumab and baricitinib have similar indications in the treatment of patients with severe COVID-19 but no head-to-head RCTs have been performed so far. Therefore, we performed a retrospective observational trial to compare the efficacy and safety of tocilizumab vs. baricitinib in hospitalized patients with COVID-19 with the need for oxygen support.
Methods
Study population and intervention
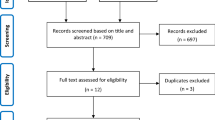
The study took place at the 4th medical department at the Klinik Favoriten in Vienna, Austria. Patients who were admitted to hospital for severe COVID-19 between 19th of February and 22nd of December 2021 and were treated with either tocilizumab or baricitinib were included in the study. Only patients with requirement for oxygen supply were treated with one of those immunomodulating drugs. All patients received dexamethasone as background medication for severe COVID-19.
The indication for tocilizumab and baricitinib in COVID-19 patients were similar at our department. Patients with a rapid disease progression (e.g., rapid deterioration from no oxygen to high-flow oxygen within 48 h), high oxygen demand on admission or patients with risk factors for disease progression (age, body-mass-index, medical history) plus elevated C-reactive protein (CRP) plus low-flow oxygen did receive tocilizumab or baricitinib. Treatment with tocilizumab required a CRP of at least 75 mg/l, while baricitinib could be prescribed at any CRP level. The decision to start an additional treatment with one of those drugs was made by the physician in charge.
Tocilizumab and baricitinib were used at different time periods throughout the pandemic at our department. Data on tocilizumab were available earlier but availability was limited later in the pandemic and it was, therefore, used from February till the 19th of September 2021. Baricitinib was used starting from 21st of September 2021 onward.
Tocilizumab dosing was based on body weight (> 90 kg: 800 mg, ≤ 90 kg: 600 mg, ≤ 65 kg: 400 mg, ≤ 40 kg: 8 mg/kg) and administered intravenously in 100 ml sodium-chloride over 1 h as a single dose regimen Baricitinib was administered orally once daily at a standard dosage of 4 mg (GFR > 60 ml/min) or 2 mg (GFR 30–60 ml/min) for up to 14 days.
Contraindications for both drugs were concomitant use of other immunosuppressive drugs (e.g., TNF alpha blockers, calcineurin inhibitors, mycophenolate mofetil, mTOR inhibitors), recent chemotherapy (within the last month), severe neutropenia (< 0.5 G/l), alanine-amino-transferase levels > 5 times upper limit of normal and suspected bacterial infection. Bacterial infections were ruled out clinically by the physician in charge before prescribing the study medication. Prior bowel perforation in history was a further contraindication for tocilizumab.
Specific contraindications for baricitinib were an estimated glomerular filtration rate < 30 ml/min, active viral hepatitis, active tuberculosis and a lymphocyte count of < 0.2 G/l. All patients were informed about the potential benefit and side effects of the study medication.
Concomitant treatment
All patients included in the study needed oxygen insufflation and, therefore, received dexamethasone as standard of care. All patients received low-molecular-weight-heparin in a prophylactic dose if no other indication for therapeutic anticoagulation (e.g., atrial fibrillation, history of pulmonary embolism or deep vein thrombosis) or contraindication was present. Remdesivir was allowed if deemed beneficial by the treating physicians. The use of monoclonal antibodies was allowed in seronegative patients. Sotrovimab and regdanvimab were available at our department during the study period.
Outcome parameters and definition of variable
In-hospital mortality was defined as the primary outcome. Progression to mechanical ventilation, length of stay (LOS) of survivors and potential side effects of the study drugs were the secondary outcome parameters.
Every patient was categorized on the seven-category ordinal scale from the World Health Organization (WHO) on admission and when treatment with tocilizumab or baricitinib was initiated. The seven-categories of the WHO proposed scale are as follows: 1. Not hospitalized, no limitations on activities; 2. Not hospitalized, limitation on activities; 3. Hospitalized, not requiring supplemental oxygen; 4. Hospitalized, requiring supplemental oxygen; 5. Hospitalized, on non-invasive ventilation or high flow oxygen devices; 6. Hospitalized, on invasive mechanical ventilation or ECMO; 7. Death.
Bacterial superinfection was defined as any suspected or proven bacterial infection which resulted in treatment with systemic antibiotics. Viral reactivation was defined as any reactivation of latent herpes or hepatitis virus infections. Elevated liver enzymes were defined as ALAT > 3 times upper limit of normal. Deep vein thrombosis or pulmonary embolism were considered as thrombotic events.
Data collection, statistical analysis and ethics approval
Data were collected retrospectively from patients records and electronic databases of the hospital. Data were anonymized before statistical analysis. All analyses were made with SPSS 25 (IBM, Armonk, NY, USA) for Mac OS (Apple, Cupertino, CA, USA). Results were expressed as relative frequencies for categorical variables, mean with standard deviations (SD) for continuous variables and median with interquartile range (IQR) for skewed distributions. Chi2-test or Fisher exact test was used for categorial variables, while t-test (or Welch test if variances were heterogen) and Mann–Whitney-U test were used for non-skewed and skewed continuous variables, respectively. A two-sided alpha < 0.05 was considered statistically significant.
The study was approved by the ethics committee of the capital city Vienna (EK 22–044). All methods were carried out in accordance with the ethical principles of the declaration of Helsinki.
Results
Patients’ characteristics on admission
The mean age was 60.5 years (SD 15.8), approximately two thirds were male with a mean BMI of 31.2 (SD 6.8). The three most common diseases in history were hypertension (48%), diabetes mellitus (27%) and coronary artery disease (14%). Comorbidities did not differ between the two groups except for a higher rate of coronary artery disease (22% vs. 8%, p = 0.009) in the tocilizumab group.
Patients in the tocilizumab group were admitted 1 day earlier after symptom onset than patients in the baricitinib group (7.4 days [SD 2.6] vs. 8.7 days [SD 3.6], p = 0.011) and had a higher median CRP level on admission (123 mg/l [IQR 103–170] vs. 89 mg/l [IQR 49–134], p = 0.001).
WHO scale at baseline was different between the two groups, with 14% having a WHO scale of 3 (10% tocilizumab vs. 16.5% baricitinib), 62% a WHO scale of 4 (55% tocilizumab vs. 67% baricitinib) and 24% a WHO scale of 5 (35% tocilizumab vs. 16.5% baricitinib), p = 0.021. For details see Table 1.
Characteristics on treatment initiation and concomitant treatment
At treatment initiation distribution of WHO scale categories was different between groups, with 48% having a WHO scale of 4 (22% tocilizumab vs. 67% baricitinib), 50% a WHO scale of 5 (72% tocilizumab vs. 33% baricitinib) and 2% a WHO scale of 6 (6% tocilizumab vs. 0% baricitinib), p < 0.001. The median CRP level was higher in the tocilizumab group (123 [IQR 107–176] vs. 90 [IQR 54–136], p < 0.001). More patients in the tocilizumab group were treated with remdesivir (44% vs. 17%., p < 0.001), while no patients received treatment with monoclonal antibodies (0% vs. 25%, p < 0.001). Treatment with tocilizumab or baricitinib was initiated after a median of 1 day after hospitalization and did not differ between groups. The average duration of baricitinib therapy was 5.98 days (SD 3.25). All patients received dexamethasone as standard of care. For details, see Table 2.
Outcome and side effects
In-hospital mortality overall was 14% and not statistically different between the two groups (tocilizumab 18% vs. baricitinib 11%, p = 0.229). There was no difference in progression to mechanical (tocilizumab 19% vs. baricitinib 11%, p = 0.173). Median length of stay of survivors was 13 days (IQR 9–18) and did not differ between groups (p = 0.114).
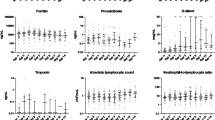
Bacterial superinfections, lymphopenia, thrombotic events and viral reactivation were equally distributed between the groups, while ALAT elevation was significantly more often observed in the tocilizumab group (43% vs 0.25%, p = 0.021). For details, see Table 3.
Discussion
In our retrospective observational trial in-hospital mortality, progression to mechanical ventilation and length of stay of survivors were not significantly different in severely ill COVID-19 patients treated with tocilizumab or baricitinib additionally to dexamethasone. The rate of side effects did neither differ, except for a significant higher rate of ALAT elevations in the tocilizumab group.
Despite similar indications of both drugs at our department the two groups showed some disparities which may have influenced the results. Patients in the tocilizumab group were hospitalized 1 day earlier, had a higher WHO category on admission and on treatment initiation. Furthermore, the CRP level was significantly higher in patients who were treated with tocilizumab. The latter one can be explained by the fact that at least a CRP of 75 mg/l was required to initiate tocilizumab treatment, like in the RECOVERY trial [24]. Taken together patients in the tocilizumab group seem to have been sicker and, therefore, may have had a higher baseline risk for a negative outcome.
More patients in the tocilizumab group have been treated with remdesivir which can be explained by the earlier admission of patients in that group. At our department remdesivir was prescribed to patients with no or low-flow oxygen within 7 days of symptom onset. Remdesivir has shown to reduce progression to mechanical ventilation and mortality in several trials [9,10,11,12,13,14,15,16] and may have modified the potential effect of tocilizumab. When patients who received remdesivir were excluded from analysis there was a significantly lower mortality and a trend toward a shorter length of stay in the baricitinib group (for details see supplemental Table 2). Possible explanations for these results may be improved outcome due to remdesivir, by chance, interaction of remdesivir and tocilizumab or selection of a special patient population.
Approximately one quarter of patients in the baricitinib group received monoclonal antibodies. The antibodies casirivimab and imdevimab were able to reduce in-hospital mortality only in seronegative (NNT = 17) but not in seropositive patients in the RECOVERY trial [25]. The monoclonal antibodies sotrovimab or regdanvimab (whatever was available) were only administered to seronegative patients at our institution. The serostatus was promptly available via our laboratory. While sotrovimab showed promising results in outpatients [26], it did not improve clinical outcome parameters in hospitalized patients [27]. To the best of our knowledge, no data about the efficacy of regdanvimab in hospitalized patients exist.
We did not detect any differences in primary and secondary outcome parameters in our whole population, but the administration of antibodies may have influenced the results as suggested by subgroup analysis in which patients who received antibodies were excluded. There was a strong trend toward a reduced mortality in the baricitinib group compared to the tocilizumab group, significant reduction of progression to mechanical ventilation and a significant shorter length of stay of survivors (for details see supplemental Table 1). Patients who did not produce antibodies around 8 days after symptom onset may have had a higher baseline risk for severe outcome and exclusion of these patients led to a better outcome of the baricitinib group.
Both tocilizumab and baricitinib were associated with positive effects on outcome in hospitalized COVID-19 patients when administered additionally to dexamethasone [21, 22, 24, 28]. Tocilizumab reduced progression to mechanical ventilation and mortality in several RCTs which was summarized in a large metanalysis [21]. The efficacy of baricitinib was first shown in one large RCT, where it failed to reduce the combined primary endpoint progression to high-flow oxygen, non-invasive ventilation, invasive ventilation or death but lead to a reduction of all-cause and 60-days mortality [22]. In a small RCT baricitinib was able to reduce 28-day and 60-day mortality of patients who were mechanically ventilated on treatment initiation, with an even lower NNT of 5 [29].
In the RECOVERY trial where baricitinib was compared to standard of care in a large multicenter RCT (N = 8156) a significant reduction of 28-days mortality from 14 to 12% was shown (NNT = 50). The effect was mostly driven by the subgroup of patients who were on non-invasive ventilation and almost all patients received dexamethasone as background medication. A meta-analysis of all other published RCTs investigating the effect of JAK inhibitors showed a larger effect on mortality reduction (10% vs. 16%, NNT = 17) [28]. Overall, both drugs seem to effectively reduce mortality in hospitalized COVID-19 patients when added to dexamethasone.
In our study in-hospital mortality, progression to mechanical ventilation and length of hospital stay were not statistically different between patients treated with tocilizumab or baricitinib. To the best of our knowledge, no head-to-head comparison of those two drugs has been performed in RCTs to date. Results from a retrospective observational trial (165 tocilizumab, 76 baricitinib) showed no differences in time to clinical improvement, discharge, in-hospital mortality and rate of side effects in hospitalized patients receiving tocilizumab or baricitinib additionally to dexamethasone [30]. In another small trial (64 tocilizumab, 34 baricitinib) both drugs did not differ in the effect on respiratory improvement nor the rate of superinfections [31]. Mortality rates were not different in an additional small trial (20 tocilizumab, 12 baricitinib) [32]. Taken together results from those trials did not find any difference in outcome parameters between patients treated with tocilizumab or baricitinib, which is in line with our study results.
Tocilizumab has the advantage of a single dose application and can be prescribed in patients with advanced kidney disease. The major disadvantages are the lack of CRP increase and the long half-life of the drug when bacterial superinfections are suspected. Baricitinib on the other hand can be taken orally and stopped in case of superinfections but cannot be prescribed in advanced chronic kidney disease. The decision for one of these drugs should be based on clinical experience, contraindications and preferred route of administration as evidence is available for both drugs.
The rate of bacterial superinfections, lymphopenia, viral reactivation and thrombotic events was not different between the two groups in our study. This is in line with the aforementioned trials [30,31,32]. RCTs did not find a higher rate of superinfections and thrombotic events in COVID-19 patients treated with tocilizumab or barictinib [21, 22, 29]. ALAT elevations of > 3 times upper limit of normal was more often reported in patients treated with tocilizumab in our study. More patients in this group were treated with remdesivir and both drugs are known for this potential side effect [33, 34]. Subgroup analysis of patients who did not receive remdesivir showed the same results (see supplemental Table 2).
The strength of our study is that we compared both drugs head-to-head which adds additional evidence in a field of interest, where only a small number of observational studies and no RCT exist. Our study has several limitations. First, we did not have a placebo group, because all patients fulfilling certain criteria were treated with an additional immunosuppressive agent. Second, it was not a randomized controlled trial, but it can be considered as “pseudorandomized” trial, because both drugs were only available during a certain time period which determined the treatment with one of the study drugs. Third, some disparities in baseline characteristics and concomitant treatment were present which may have influenced the outcome parameters. Fourth, no data about the virus subtypes are available. Patients treated with tocilizumab may have had infections caused by the alpha and delta strain, while patients in the baricitinib were infected mainly with the delta strain, because those strains were circulating at the study period [35]. Fifth, no omicron patients were included in our study.
In summary, in-hospital mortality, progression to mechanical ventilation and length-of-stay were not different in hospitalized severely ill COVID-19 patients treated with tocilizumab or baricitinib additionally to dexamethasone in our retrospective observational study. Further except for a higher rate of liver enzyme elevation in the tocilizumab group, side effects did not differ between the two treatment groups. Multiple RCTs have proven the beneficial effect of those drugs [21, 22, 24, 28, 29] and its use additionally to corticosteroids is recommended in several guidelines [1, 2]. To the best of our knowledge no RCT exists which compares both drugs head-to-head, so our study adds valuable evidence that both drugs may be equally effective as shown by only a handful of retrospective small trials [30,31,32]. Further large studies are needed to confirm these results.
Availability of data and materials
Data will be made available if requested.
References
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [13th of June, 2022].
Therapeutics and COVID-19: Living guideline, 22 April 2022. Geneva: World Health Organization; 2022 (WHO/2019-nCoV/therapeutics/2022.3). Licence: CC BY-NC-SA 3.0 IGO.
Blair JE, Gotimukul A, Wang F, et al. Mild to moderate COVID-19 illness in adult outpatients: characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine (Baltimore). 2021;100: e26371. https://doi.org/10.1097/MD.0000000000026371.
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55: 105924. https://doi.org/10.1016/j.ijantimicag.2020.105924.
Rudiansyah M, Jasim SA, Mohammad Pour ZG, et al. Coronavirus disease 2019 (COVID-19) update: from metabolic reprogramming to immunometabolism [published online ahead of print, 2022 Jun 10]. J Med Virol. 2022. https://doi.org/10.1002/jmv.27929.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20. https://doi.org/10.1056/NEJMoa2116044.
Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–408. https://doi.org/10.1056/NEJMoa2118542.
Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–15. https://doi.org/10.1056/NEJMoa2116846.
Marx K, Gončarova K, Fedders D, et al. Clinical outcomes of hospitalized COVID-19 patients treated with remdesivir: a retrospective analysis of a large tertiary care center in Germany [published online ahead of print, 2022 May 12]. Infection. 2022. https://doi.org/10.1007/s15010-022-01841-8.
Olender SA, Walunas TL, Martinez E, et al. Remdesivir versus standard-of-care for severe coronavirus disease 2019 infection: an analysis of 28-day mortality. Open Forum Infect Dis. 2021;8:ofab278. https://doi.org/10.1093/ofid/ofab278 (Published 2021 May 26).
Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort [published online ahead of print, 2021 Oct 1]. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab875.
Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76:3296–302. https://doi.org/10.1093/jac/dkab321.
WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399:1941–53. https://doi.org/10.1016/S0140-6736(22)00519-0.
Ader F, Bouscambert-Duchamp M, Hites M, et al. Final results of the DisCoVeRy trial of remdesivir for patients admitted to hospital with COVID-19. Lancet Infect Dis. 2022;22:764–5. https://doi.org/10.1016/S1473-3099(22)00295-X.
Ali K, Azher T, Baqi M, et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–51. https://doi.org/10.1503/cmaj.211698.
Kaka AS, MacDonald R, Linskens EJ, et al. Major update 2: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2022;175:701–9. https://doi.org/10.7326/M21-4784.
Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–6. https://doi.org/10.1002/jmv.26232.
Pacheco-Hernández LM, Ramírez-Noyola JA, Gómez-García IA, Ignacio-Cortés S, Zúñiga J, Choreño-Parra JA. Comparing the cytokine storms of COVID-19 and pandemic influenza [published online ahead of print, 2022 Jun 7]. J Interferon Cytokine Res. 2022. https://doi.org/10.1089/jir.2022.0029.
Bahmani M, Chegini R, Ghanbari E, et al. Severe acute respiratory syndrome coronavirus 2 infection: role of interleukin-6 and the inflammatory cascade. World J Virol. 2022;11:113–28. https://doi.org/10.5501/wjv.v11.i3.113.
RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with COVID-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. https://doi.org/10.1001/jama.2021.11330.
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published correction appears in Lancet Respir Med. 2021 Oct;9(10):e102]. Lancet Respir Med. 2021;9:1407–18. https://doi.org/10.1016/S2213-2600(21)00331-3.
Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;385:406–15. https://doi.org/10.1056/NEJMoa2101643.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45. https://doi.org/10.1016/S0140-6736(21)00676-0.
RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–76. https://doi.org/10.1016/S0140-6736(22)00163-5.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50. https://doi.org/10.1056/NEJMoa2107934.
ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622–35. https://doi.org/10.1016/S1473-3099(21)00751-9.
RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. 2022;400:359–68. https://doi.org/10.1016/S0140-6736(22)01109-6.
Ely EW, Ramanan AV, Kartman CE, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial [published correction appears in Lancet Respir Med. 2022 Feb 11]. Lancet Respir Med. 2022;10:327–36. https://doi.org/10.1016/S2213-2600(22)00006-6.
Wong CKH, Lau KTK, Au ICH, et al. Initiation of Tocilizumab or Baricitinib were associated with comparable clinical outcomes among patients hospitalized with COVID-19 and treated with dexamethasone. Front Pharmacol. 2022;13: 866441. https://doi.org/10.3389/fphar.2022.866441 (Published 2022 May 30).
Kojima Y, Nakakubo S, Takei N, et al. Comparative efficacy of Tocilizumab and Baricitinib administration in COVID-19 treatment: a retrospective cohort study. Medicina (Kaunas). 2022;58:513. https://doi.org/10.3390/medicina58040513 (Published 2022 Apr 4).
Rosas J, Liaño FP, Cantó ML, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol Clin (Engl Ed). 2022;18:150–6. https://doi.org/10.1016/j.reumae.2020.10.006.
https://www.gilead.com/-/media/files/pdfs/medicines/COVID-19/veklury/veklury_pi.pdf (accessed 27th of June 2022)
https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf (accessed 27th of June 2022)
https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus#c12422 (accessed 14th of June 2022)
Acknowledgements
Data were collected as a part of the diploma thesis of the author Saak Magdalena.
Funding
This work did not receive any funding.
Author information
Authors and Affiliations
Contributions
MK, EP, SO and AG had the idea of the study. MK wrote the manuscript. MK, MT and MS analyzed the data. EP, TS, WH and HL proofread the manuscript. AG, MS, DP, JS, AK, CO and HK collected the data. AA, CW and AZ supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare that they do not have any conflicts of interest.
Ethical approval
The study was approved by the ethics committee of the capital city Vienna.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karolyi, M., Gruebl, A., Omid, S. et al. Tocilizumab vs. baricitinib in hospitalized severe COVID-19 patients: results from a real-world cohort. Infection 51, 851–858 (2023). https://doi.org/10.1007/s15010-022-01915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01915-7