Abstract
Echinocandins represent the first-line therapy of candidemia. Echinocandin resistance among Candida spp. is mainly due to acquired FKS mutations. In this study, we report the emergence of FKS-mutant Candida albicans/glabrata in Switzerland and provide the microbiological and clinical characteristics of 9 candidemic episodes. All patients were previously exposed to echinocandins (median 26 days; range 15–77). Five patients received initial echinocandin therapy with persistent candidemia in 4 of them. Overall mortality was 33%.
Similar content being viewed by others
Introduction
The pathogenic yeasts Candida spp. are an important cause of nosocomial bloodstream infections, which are associated with high mortality rates [1]. Candida albicans represents the most frequent cause of candidemia, but a progressive epidemiological shift towards more resistant non-albicans Candida spp. (e.g., Candida glabrata) is reported all over the world [1]. In this context, echinocandins (e.g., anidulafungin, caspofungin and micafungin) have become the first-line antifungal therapy because of their better efficacy and broader antifungal spectrum against Candida spp. compared to azoles [2]. However, increased use of echinocandins has been associated with the emergence of resistance to these drugs, which affects particularly C. albicans and C. glabrata [3]. Echinocandin resistance in Candida spp. results from well-defined mutations in hotspot regions of the FKS gene that encodes for the 1,3-beta-d-beta-glucan synthase, the target of echinocandins [4]. These mutations usually result in pan-echinocandin resistance and affect mainly C. glabrata (prevalence range 2–13%) and more rarely C. albicans (< 1%) [3, 5,6,7,8,9]. Previous echinocandin exposure has been identified as the main risk factor and was observed in most cases [3, 5]. Because of the limited therapeutic alternatives, in particular for C. glabrata candidemia, mortality rates are high (60%) [5]. In Switzerland, a recent nationwide survey of candidemia (2004–2013) reported a very low rate of FKS-mutant C. albicans and C. glabrata (2 out of 1624 isolates, 0.12%) [10]. However, several echinocandin-resistant isolates harbouring various types of FKS mutations have been reported from different centers since that time. The aim of this study was to characterize these strains and to report the clinical characteristics and outcome of their associated candidemic episodes.
Materials and methods
Patients and data collection
Clinical isolates of candidemic C. albicans and C. glabrata that were non-susceptible to micafungin and/or anidulafungin according to the clinical breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) [11] were retrospectively identified by (i) screening of a nationwide survey of candidemia by the Fungal Infection Network of Switzerland (FUNGINOS) conducted from 2004 to 2013 in 25 university and university-affiliated medical centers of Switzerland, and (ii) a call to the microbiologists of the university hospitals to screen and send their candidemic Candida spp. isolates with phenotypic echinocandin resistance to the reference laboratory (Lausanne University Hospital) for the period 2013–2019. Demographic characteristics and clinical data were collected via a standard clinical report form (CRF) including underlying conditions, risk factors for candidemia, previous courses of antifungal therapy within the last 3 months, clinical characteristics, treatment and outcome of Candida infection.
Antifungal susceptibility testing and sequencing
All Candida isolates were handled in our reference laboratory. Minimum inhibitory concentrations (MIC) of anidulafungin, micafungin, caspofungin and fluconazole were retested in duplicates by microbroth dilution method according to the protocol M27 (4th edition) of the CLSI [11]. Susceptibility (S), dose-dependent susceptibility (SDD) or resistance (R) were defined according to the CLSI breakpoints, which are for anidulafungin and caspofungin (in µg/mL): C. albicans S ≤ 0.25, R ≥ 1, C. glabrata S ≤ 0.12, R ≥ 0.5, for micafungin: C. albicans S ≤ 0.25, R ≥ 1, C. glabrata S ≤ 0.06, R ≥ 0.25, and for fluconazole: C. albicans S ≤ 2, R ≥ 8, C. glabrata SDD ≤ 32, R ≥ 64. Isolates for which MICs were confirmed to be above the susceptibility clinical breakpoints (i.e., classified as non-susceptible according to the CLSI breakpoints) for micafungin and/or anidulafungin were selected for genotypic characterization of FKS genes (of note, isolated elevated caspofungin MIC was not considered as a reliable predictor of FKS mutations on the basis of previous publications [10, 12]). PCR and sequencing of the hotspot regions (HS) of the FKS genes (HS1 and HS2 of FKS1 for C. albicans and C. glabrata and HS1 and HS2 of FKS2 for C. glabrata) were performed using the primers previously described [10].
Ethical statement
This study was approved by the Swiss ethics committee on research involving humans for retrospective use of clinical data (Swissethics reference # 2019-00484_1904).
Results
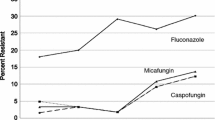
A total of 9 cases of candidemia due to echinocandin-resistant Candida spp. (4 C. albicans and 5 C. glabrata) were identified. Two cases with confirmed FKS mutations were obtained from the FUNGINOS nationwide survey from 2004 to 2013. Seven additional cases were reported from 4 hospitals from 2013 to 2019. In total, 5 of the 9 cases were observed within the last 2 years (2018–2019). For 4 cases, a susceptible isolate of the same Candida species was obtained from previous blood cultures (4–19 days before the candidemic episode attributed to the resistant isolate). MIC of anidulafungin, micafungin, caspofungin and fluconazole, as well as results of FKS hotspots sequencing for all the 9 cases (13 isolates, including the 9 echinocandin-resistant strains and the 4 previously susceptible strains) are shown in Table 1. The most frequent mutations were S645P (n = 3) for C. albicans and S663P in FKS2 (n = 3) for C. glabrata. The 3 remaining echinocandin-resistant isolates were one C. albicans with R1361G mutation and two C. glabrata isolates with S629P (FKS1) and F659_(FKS2) mutations, respectively. All 9 isolates were classified as non-susceptible to all three echinocandins according to CLSI breakpoints. One of the four echinocandin-resistant C. albicans isolates and all C. glabrata isolates were classified as susceptible dose-dependent to fluconazole. Absence of FKS mutations was confirmed for the 4 susceptible isolates obtained from the previous candidemic episodes.
Clinical characteristics of the 9 cases are shown in Table 2. All patients have been exposed to echinocandins before the candidemic episode with the resistant isolate (median duration: 26 days, range 15–77). Five episodes were breakthrough candidemia (i.e., occurring while echinocandin treatment was ongoing). Five cases had a deep focus of infection (3 abdominal and 2 urinary), while candidemia was primary or originating from a catheter in 4 cases. Five patients received initial echinocandin therapy (duration 7–18 days) with persistent candidemia (duration between first and last positive blood cultures: 5–40 days) in 4 of them. In these cases, candidemia was cleared after switch to another antifungal drug (liposomal amphotericin B in most cases). Three of the nine patients died within 6 weeks from the candidemia and death was at least partially attributed to candidemia (i.e., active disease at time of death) in two cases.
Discussion
In this study, we described the microbiological and clinical characteristics of nine episodes of candidemia due to FKS-mutant C. albicans/glabrata that were observed in Switzerland between 2004 and 2019. The actual prevalence of these FKS mutations among C. albicans/glabrata candidemic episodes was assessed for the period 2004–2013 via a national surveillance program of the Fungal Infection Network of Switzerland (FUNGINOS) collecting all candidemic isolates from 25 Swiss medical centers (including all university and most university-affiliated hospitals) [10]. Only 2 FKS-mutant isolates were recovered from this 10-year period (prevalence 0.12%). We could not assess the prevalence for the period 20,014–2019, where only isolates with phenotypic resistance from a limited subset of university hospitals were sent to our laboratory for FKS sequencing. However, the fact that we identified 7 FKS-mutant C. albicans/glabrata from 4 different Swiss centers over this 6-year period suggests an increase of the rate of FKS-mutant echinocandin resistant isolates. The incidence of candidemia remained relatively stable in Switzerland over the last decade according to our national surveillance systems (unpublished data), but we have observed a constant increase of echinocandin consumption since 2004, as previously reported [10]. Echinocandin pre-exposure was previously identified as the main risk factor for FKS mutations [3, 5]. In our study, all patients received previous echinocandin treatment for > 15 days, which is in keeping with previous reports (median 3–4 weeks) [3, 5]. Caspofungin is the most used echinocandin drug in Switzerland and a recent publication suggests that it is associated with a higher risk of inducing FKS mutations in comparison to other echinocandins [13]. Other risk factors of acquired FKS mutations have been suggested, such as the existence of hidden reservoirs or uncontrolled sources of infection [14], which was probably the case for more than half (5/9) patients of our series with complicated intra-abdominal candidiasis or persistent urinary tract colonization. Among the 5 patients who received initial echinocandin therapy fo R ≥ 7 days, 4 failed to respond (i.e., persistent candidemia under echinocandin therapy) and ultimately survived after switch for another antifungal drug. The patient who responded to echinocandin therapy despite high MIC values had an intravascular catheter infection and was possibly cured by catheter removal only. The cases for which echinocandin therapy was initially continued, despite resistance to this antifungal drug class, were mainly patients with severe comorbidities (including renal dysfunction) who were infected with C. glabrata, a species exhibiting some degree of natural resistance to azoles. This outlines the difficulty to treat such infections because of the lack of therapeutic alternatives. Amphotericin B formulations often remain the unique option and their use could be limited by renal toxicity. However, our results clearly suggest that echinocandin therapy should not be continued against these FKS-mutant Candida species, even in those with moderate MIC elevation (e.g., 2–4 µg/mL), and is associated with failure to therapy. Overall mortality was 33%, which is in the usual ranges of mortality rates that have been reported for candidemia in general [1].
FKS mutations observed in our study were diverse, but predominantly S645P in C. albicans and S663P (FKS2) in C. glabrata. All FKS-mutant isolates of our study exhibited echinocandin MICs that were classified as non-susceptible according to the CLSI breakpoints. However, MIC values varied between 0.25 and > 16 µg/mL with caspofungin MIC being usually higher compared to anidulafungin or micafungin MICs.
Echinocandin resistance among Candida spp. remains rare. Previous observations are limited to small case-series (7–25 cases) with sometimes incomplete clinical data and mainly reported from the North American continent [3, 5, 6, 8, 9]. The present report of these 9 cases suggests that echinocandin resistance due to acquired FKS mutations is an emerging concern in Europe. These cases were associated with failure of echinocandin therapy, which is particularly concerning for C. glabrata due to the limited therapeutic options. Echinocandin resistance should be suspected in patients with previous echinocandin exposure (> 15 days), particularly in the presence of hidden or uncontrolled reservoirs, such as abdominal candidiasis or urinary tract colonization.
Availability of data and material
The sequences of FKS hotspots regions of all Candida isolates of this study have been deposited at the National Center for Biotechnology Information (NCBI) under Accession Numbers MT396587 to MT396628.
References
Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73:i4–i13. https://doi.org/10.1093/jac/dkx444.
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37. https://doi.org/10.1111/1469-0691.12039.
Shields RK, Nguyen MH, Press EG, Cumbie R, Driscoll E, Pasculle AW, et al. Rate of FKS Mutations among Consecutive Candida Isolates Causing Bloodstream Infection. Antimicrob Agents Chemother. 2015;59:7465–70. https://doi.org/10.1128/AAC.01973-15.
Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat. 2013;16:81–95.
Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis. 2014;59:819–25. https://doi.org/10.1093/cid/ciu407.
Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. Anidulafungin and micafungin MIC breakpoints are superior to that of caspofungin for identifying FKS mutant Candida glabrata strains and Echinocandin resistance. Antimicrob Agents Chemother. 2013;57:6361–5. https://doi.org/10.1128/AAC.01451-13.
Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, et al. Update from a 12-Year Nationwide Fungemia Surveillance: Increasing Intrinsic and Acquired Resistance Causes Concern. J Clin Microbiol. 2018. https://doi.org/10.1128/JCM.01564-17.
Fuller J, Dingle TC, Bull A, Shokoples S, Laverdiere M, Baxter MR, et al. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011–16 study. J Antimicrob Chemother. 2019;74:iv48–iv54. https://doi.org/10.1093/jac/dkz287.
Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56(12):1724–32. https://doi.org/10.1093/cid/cit136.
Kritikos A, Neofytos D, Khanna N, Schreiber PW, Boggian K, Bille J, et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: a 10 year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect. 1214e;24:1214e1–e4. https://doi.org/10.1016/j.cmi.2018.05.012.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts (4th ed.), CLSI, Wayne, PA (M27-Ed4). 2017.
Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, et al. Interlaboratory variability of Caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother. 2013;57:5836–42. https://doi.org/10.1128/AAC.01519-13.
Shields RK, Kline EG, Healey KR, Kordalewska M, Perlin DS, Nguyen MH, et al. Spontaneous Mutational Frequency and FKS Mutation Rates Vary by Echinocandin Agent against Candida glabrata. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/AAC.01692-18.
Shields RK, Nguyen MH, Clancy CJ. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis. 2015;28(6):514–22. https://doi.org/10.1097/QCO.0000000000000215.
Acknowledgements
Open access funding provided by University of Lausanne.
Funding
This study was funded by the Fungal Infection Network of Switzerland (FUNGINOS).
Author information
Authors and Affiliations
Consortia
Contributions
ATC: laboratory testing, data collection, data analyses, redaction of manuscript, AK: data collection, data analyses, redaction of manuscript, LJ: laboratory testing, data analyses, NK: data collection, review of manuscript, DG: data collection, review of manuscript,, CG: data collection, review of manuscript, CZ: data collection, review of manuscript, KB: data collection, review of manuscript, DN: data collection, review of manuscript, AR: data collection, review of manuscript, DB: laboratory testing; DS: data analyses, review of manuscript, FL: study design, data analyses, redaction of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author Zehnder C. was employed by company SYNLAB Suisse SA, Bioggio, Switzerland. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coste, A.T., Kritikos, A., Li, J. et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 48, 761–766 (2020). https://doi.org/10.1007/s15010-020-01475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-020-01475-8