Abstract
Background:
HIV-associated lipodystrophy syndrome (LDS) as a long-term side effect of HAART is becoming increasingly important and negatively affects adherence to medication. Currently, an effective therapy is not available. There is some evidence that the drug class of thiazolidindiones might be effective in the treatment of LDS.
Patients and Methods:
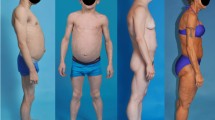
Prospective open-label study with 20 HIV-infected patients suffering from severe LDS. Patients received 4 mg rosiglitazone once daily for a 24-week study period. Efficacy was assessed by measurement of metabolic and anthropometric parameters, total body DXA scan, CT scan of the abdomen, photo documentation and self-assessment.
Results:
Rosiglitazone treatment was well tolerated. DXA scans demonstrated a highly significant increase in adipose tissue of the limbs (2644 ± 1334 g vs 3380 ± 1614 g, p ≤ 0.001) without any change in total fat mass. Abdominal CT-scans revealed a significant increase in subcutaneous adipose tissue (113.7 ± 82.4cm2 vs 125.3 ± 83.7 cm2, p = 0.04). Abdominal circumference decreased significantly (94.7 ± 8.7 cm vs 92.2 ± 8.45 cm, p = 0.03) without any relevant change of body weight or BMI. We observed an increase in serum cholesterol (248 vs 281 mg/dl, p = 0.006) and serum triglycerides (301 vs 351 mg/dl, p = 0.1). Furthermore, no side effects of clinical relevance were observed. The insulin sensitivity index improved without reaching statistical significance. Thirteen patients (65%) reported general improvement of LDS symptoms. Evaluation of photo documentation by five HIV-experts revealed poor concordance and no relevant change of LDS.
Conclusions:
The results of this study suggest that rosiglitazone is safe in the treatment of HAART-associated lipodystrophy and has moderate clinical efficacy. We found a trend towards improved insulin sensitivity and as a possible limiting factor an unfavorable increase in serum cholesterol and triglycerides.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feldt, T., Oette, M., Kroidl, A. et al. Evaluation of Safety and Efficacy of Rosiglitazone in the Treatment of HIV-Associated Lipodystrophy Syndrome. Infection 34, 55–61 (2006). https://doi.org/10.1007/s15010-006-5022-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s15010-006-5022-y