Abstract
Background:
Due to its high water content and biomimetic properties simulating extracellular matrix (ECM), hydrogels have been used as preferred cell culture and delivery systems. Similarly, cell-loaded hydrogels can be easily injected into target areas in a minimally invasive manner, minimizing surgical trauma, adapting to irregular shaped defects, and benefiting patients. In this study, we systematically reviewed multiple studies on hydrogel-based bone defect research and briefly summarized the progress of injectable and cell-loaded hydrogels in bone defect repair.
Methods:
A systematic search was conducted in the PubMed and Web of Science databases using selected search terms.
Results:
Initially, 185 articles were retrieved from the databases. After full-text screening based on inclusion and exclusion criteria, 26 articles were included in this systematic review. Data collected from each study included culture model, seed cell type and origin, cell concentration, scaffold material, scaffold shape, experimental animal and site, bioactive agents, and binding method. This injectable and cell-loaded hydrogel shows certain feasibility in bone tissue engineering applications.
Conclusion:
Injectable and cell-loaded hydrogels have been widely applied in bone tissue engineering research. The future direction of bone tissue engineering for bone defect treatment involves the use of new hydrogel materials and biochemical stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the increasing occurrence of trauma, tumors, abnormalities, and infections requiring surgical intervention or treatment presents ongoing challenges in the field of orthopedics [1]. Current treatment focus remains on the “gold standard” treatments such as autologous or allogeneic grafts. However, these methods are limited by issues such as limited supply, disease transmission from donor sites, and adverse immune reactions [2, 3]. Consequently, other alternative substitutes are needed nowadays.
Thus, inorganic bone materials like synthetic and metallic substitutes have been applied in the clinic with the advantage of unlimited supply and biocompatibility [4]. However, the incapability of repairing commonly irregular-shape defects and delivering stem cells hamper the wide application of these substitutes.
Although calcium phosphate or polymethyl methacrylate (PMMA) cement can fill irregular-shape defects, the high density and exothermic reactions of bone cement restrict the delivery of regenerative cells and growth factors.
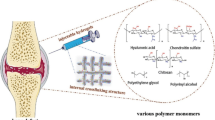
Injectable hydrogels, as a cell carrier increasingly emphasized in tissue engineering and regenerative medicine, may overcome these limitations. Hydrogels have high water content and biomimetic properties simulating the extracellular matrix, making them suitable for preferred cell culture and delivery systems. Additionally, cell-loaded hydrogels can be easily injected into target areas in a minimally invasive manner, adapting to irregular shaped defects, and benefiting patients.
In this study, we systematically reviewed multiple studies on hydrogel-based bone defect research and briefly summarized the progress of injectable and cell-loaded hydrogels in bone defect repair. This review further highlights the content of different cell-loaded hydrogel materials and attempts to answer the following questions: what types of hydrogels have been used? What cells and growth factors have been loaded into hydrogels and have potential applications?
2 Materials and methods
2.1 Search strategy
A systematic search was conducted in the PubMed and Web of Science databases using selected search terms. The study was limited to articles written in English.
2.2 Search terms
The following terms included Medical Subject Headings (MeSH) terms and free text phrases: “hydrogel” or “In Situ Hydrogels” or “In Situ Hydrogel” or “Hydrogel, In Situ” or “Patterned Hydrogels” or “Patterned Hydrogel” or “Hydrogel, Patternel”; “bone defect” or “bone loss” or “defect” or “deficiency”; and “injection”. This query aimed to find studies investigating injectable and cell-loaded hydrogels and reporting their potential in bone tissue regeneration.
2.3 Study selection
The entire literature search process was independently performed by two reviewers. Any disagreements were reviewed by a third reviewer. The following inclusion and exclusion criteria were created to determine study eligibility:
2.3.1 Inclusion criteria
(1) Studies repairing bone defects using injectable and cell-loaded hydrogels; (2) Original articles written in English only.
2.3.2 Exclusion criteria
(1) Human studies only; (2) Reviews, comments, case reports, guidelines, and technical reports; (3) Full texts not available.
2.4 Data extraction
From the included studies, the following study information was recorded: (1) Study characteristics (author, year of publication, and journal name); (2) Intervention details (hydrogel and cells used, growth factors, cross-linking materials and methods, and experimental animals); (3) Mechanical and biological properties.
3 Results
The entire literature search, inclusion, and exclusion process is shown in Fig. 1. Initially, 185 articles were retrieved from the databases. After deduplication (n = 61), 124 articles were screened for their titles and abstracts. As a result, 43 articles were selected for full-text review. The remaining articles included 9 unrelated to stem cells, 4 related to cartilage, 2 were reviews, 2 focused on signaling pathways. Ultimately, this systematic review included 26 articles. Three articles were in vitro studies 5,6,7], three were in vivo studies [8,9,10], and 20 articles were conducted simultaneously [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Table 1 summarizes the culture model, seed cell type, source, and seeding density. Table 2 summarizes the contents of hydrogels and scaffolds, experimental animal types and defect sites, and defect sizes. Table 3 presents the basic information regarding hydrogel materials, seed cell types, bioactive agents, and binding methods.
In these 26 studies, sodium alginate gel was used the most frequently [5, 8, 12, 15, 16, 20, 21, 27, 28], followed by hyaluronic acid (HA) [11, 15, 16, 30] gelatin [7, 21, 27], chitosan [11, 22, 30], and other natural and synthetic polymers. Most articles applied more than one type of hydrogel.
Several studies have investigated the delivery of stem cells via hydrogel microspheres, which offer numerous advantages over conventional bulk hydrogels such as rapid production and size controllability [20, 23, 24]. Various novel functional nanoparticles (NPs) have also been integrated into crosslinkable hydrogel networks to provide desired functionality in some studies [6, 10, 16, 19, 22, 24, 29]. Additionally, bioceramics, including hydroxyapatite, laponite, and β-tricalcium phosphate, have been employed to improve mechanical properties and decrease degradation rates in bone regeneration research. [6, 7, 10, 11, 15, 19, 22, 25, 29, 30].
Of the studies reviewed, two studies used preosteoblasts [6, 8], 23 utilized stem cells for bone differentiation, with MSCs being the most commonly used seeding cell [5, 5,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30], while another investigated co-culturing MSCs with endothelial cells [7]. Regarding the source of seed cells, rat/mouse cells were used in 15 studies [6,7,8, 10,11,12,13,14, 19,20,21, 23, 24, 28, 29], human cells in five studies [5, 15, 17, 24, 27], rabbit cells in four studies [18, 22, 27, 30], and goat/ovine cells in two studies [9, 16]. Furthermore, the density of seed cells was around 1–240 × 105 cells/ml in most cases.
Localized and sustained introduction of BMPs is used in four studies to promote robust bone tissue formation [8, 15, 17, 22], Vascularization is vital in bone regeneration, and some studies have introduced angiogenic factors into their systems to enhance bone regeneration [7, 22]. Localized gene delivery is an effective and safe alternative for growth factor delivery, as it can be achieved through several methods [7, 15, 22]. And it can be achieved by several ways, including both viral [17] and nonviral [8].
Rats were the most commonly used laboratory animals. As hydrogels do not provide the mechanical robustness required for load-bearing applications, the cranial defect module was used most frequently [7, 12,13,14, 19, 20, 24, 25, 27, 29, 30], followed by the tibia [9, 20, 26] and femur.
4 Discussion
For the therapy of bone defects, the tissue engineering approach is now a popular strategy combining the injectable hydrogel, seed cells, and growth factors for functional reconstruction in a minimally invasive way.
Based on a comprehensive literature review, our research mainly focus on the above three elements of tissue engineering and provide potential guidelines for future studies and the clinical application of stem cells and biomaterial-based bone regeneration.
4.1 Hydrogels for reconstruction of bone defects
4.1.1 Alginate hydrogels and their derivatives
Alginate is a naturally-extracted polysaccharide from brown algae containing glucuronic and mannuronic acids, which make it more selective for binding to Ca2+ ions to form hydrogel. It has been applied in numerous tissue engineering experiments due to its favorable properties including biocompatibility, biodegradability, and facile gelation process. Furthermore, their ability to encapsulate MSCs or bioactive components suggested that this hydrogel is a promising suitable injectable polymer for controlled delivery and bone tissue engineering. ChuanfengAn etc. employed alginate hydrogel beads for microencapsulation of MSCs to support cell proliferation and osteoblastic differentiation [31]. However, alginate hydrogel has many limitations for tissue engineering. First, its poor mechanical properties and short-term stability do not ensure maintenance of the regenerated tissue. Second, its poor cell adhesion properties provide limited support for cellular functionality. For this reason, alginate is often used in a chemically modified form. Ganesh C. Ingavle etc. modified the alginate with the RGD peptide sequence as adhesion ligands that can promote cell attachment, proliferation, and bone formation [16].
4.1.2 Chitosan hydrogels and their derivatives
Chitosan is an amino polysaccharide derived from crab and shrimp shells, composed of repeating units of glucosamine and N-acetylglucosamine linked by β − (1–4) glycosidic bonds. Due to its low immunogenicity, chitosan-based hydrogels have shown significant potential for promoting cell proliferation and adhesion, and have displayed great potential for bone tissue regeneration [16]. However, the mechanical strength, degradation, and osteogenic activity of pure chitosan are poor, which can affect the final repair effect. To improve its mechanical performance and biological activity, chitosan-based hydrogels need to be combined with other synthetic or natural polymers and bioactive molecules to construct multifunctional biomaterials.
4.1.3 Hyaluronic acid hydrogels and their derivatives
Hyaluronic acid is a copolymer of D-glucuronic acid and N-acetyl-D-glucosamine, which is present in all tissues. The biodegradability, cell adhesion, migration, proliferation, and differentiation properties of hyaluronic acid are important for its potential use as a tissue engineering construct [32]. Moreover, because hyaluronic acid can be enzymatically degraded, it does not cause an immune reaction and can serve as a carrier for injection. In vivo, hyaluronic acid can promote the construction of a matrix around cells, providing a suitable microenvironment for stem cell differentiation. Additionally, hyaluronic acid hydrogels are commonly used as carriers for growth factors, such as BMPs, to promote osteogenesis. However, the mechanical performance of hyaluronic acid hydrogels is poor. To overcome these drawbacks, a range of modification methods has been developed. Chemical modification of hyaluronic acid can be achieved by reacting its carboxylic groups with various hydroxyl or amine-containing groups to form derivatives with better biocompatibility and controllable degradation [32].
4.1.4 Gelatin hydrogels and their derivatives
Gelatin is a hydrolysis product extracted from collagen, which is the main component of cartilage tissue extracellular matrix (ECM). Gelatin has excellent cell adhesion, biocompatibility, and biodegradability properties. However, it has a drawback in that its physical crosslinking stability is low in vivo. Therefore, chemical modification of gelatin is required before use in gelatin hydrogels [31]. GelMA retains most of the functional amino acid groups of gelatin, thus possessing excellent cell adhesion properties. Due to its inherent biological activity and tunable physicochemical properties, GelMA has been widely used in tissue engineering applications. Although these hydrogels are easy to adapt to microfluidic technology [33], they also have some drawbacks. The free radicals released during photodependent crosslinking, residual monomers and photoinitiators in hydrogel microparticles have high cell toxicity [34]. Moreover, the crosslinking strength cannot be controlled during this process, inadequate crosslinking may lead to microsphere fusion and rupture, while excessive crosslinking will greatly reduce the internal porosity of the microspheres, affecting cell behavior. Finally, excessive ultraviolet irradiation during the crosslinking process may reduce cell survival.
4.1.5 Typical synthetic (composite) polymer-based hydrogels
The main drawbacks of natural hydrogels include poor mechanical properties, fast and unpredictable degradation, and strong dependence on the individual, enzyme levels, and injection site. Chemical modifications of hydrogels, such as the addition of specific functional groups and macromolecules, can overcome their inherent limitations by improving their mechanical properties and adjusting their biodegradability [35]. Synthetic hydrogels have great potential for use in regenerative medicine due to their programmable and reproducible properties [36]. Poly(ethylene glycol) (PEG), poly(2-hydroxyethyl methacrylate) (PHEMA), and polyvinyl alcohol (PVA) are the most commonly used hydrogels in the biomedical field because of their high hydrophilicity, non-toxicity, and ease of functionalization through chemical reactions. However, most hydrogels are usually weak and easily degraded under physiological conditions [37]. To overcome this drawback, a special class of interpenetrating network (IPN) polymers, called double network (DN) hydrogels, has recently been synthesized to address issues such as rapid crosslinking, injectability, and cell compatibility.
4.2 Various types of cells encapsulated in hydrogels
4.2.1 Preosteoblasts and osteoblasts
Pre-osteoblasts are good cell sources for bone tissue engineering. Although stem cells have self-renewal and pluripotent differentiation abilities, making them a better source. Pre-osteoblasts have stronger osteogenic properties than MSCs, so they still have good research and application prospects.
4.2.2 Stem cells
MSCs are important tools in regenerative medicine due to their chemotaxis, multidirectional differentiation, and immunomodulatory capabilities [38]. In recent years, MSCs including BMSCs, ADSCs, and hESCs have been widely studied. They all have some osteogenic ability in hydrogels both in vitro and in vivo.
BMSCs are a cell source for many bone tissue engineering applications because they have higher proliferation capacity and are generally easier to obtain than mature osteoblasts [39].
ADSCs are widely used as seed cells for tissue engineering due to their ease of acquisition, strong proliferative activity, multipotent potential, and immunomodulatory capability [40].hESCs are also a very promising source of cells because they have long-term proliferation and self-renewal abilities and can differentiate into almost all cell types [41].
4.2.3 Co-culture of endothelial cells and stem cells
Bone is a highly vascularized tissue, and osteogenesis and angiogenesis are coupled. There is a synergistic effect between bone cells and endothelial cells during bone regeneration. When MSCs and endothelial cells are co-cultured, the markers for osteogenesis and blood vessel generation are enhanced compared to single culture of the cells. On the other hand, vascular injury can inhibit bone growth and induce skeletal diseases [42]. In the construction of synthetic biomaterials for bone regeneration, insufficient formation of a vascular network can affect bone healing and even lead to tissue necrosis. However, developing vascularized bone implants remains a challenge until now.
4.3 Growth factors on hydrogels
In the natural process of bone defect repair, MSCs and many other types of cells interact with growth factors, among which bone morphogenetic protein-2 (BMP-2) plays a dominant role in promoting bone defect healing due to its strong osteogenic properties. Vascular endothelial growth factor (VEGF) is the most important growth factor for promoting angiogenesis and new bone formation in vivo [43].
4.3.1 BMP-2
BMP-2 is one of the BMPs that have been shown to have strong osteoinductive effects [44]. The molecule is involved in the osteoinductive signaling pathway, promoting differentiation of MSCs into osteoblasts. BMP-2 has great potential in bone regeneration, but a large amount of protein is required to produce an effect due to the degradability of proteins in vivo [45].
4.3.2 VEGF
Vascularization is a key process in bone regeneration and a limiting step in the healing of large area bone defects [46, 47]. In the past decade, hydrogels have been functionalized by loading biologically active molecules into drug delivery systems, forcing them to locally deliver at the required time in sufficient doses. Among them, vascular endothelial growth factor can maintain long-term stability and half-life with few adverse reactions. In this case, hydrogels wrapped with these biologically active molecules can regulate and promote cell differentiation, proliferation, migration, and vascularization.
5 Conclusion
Based on the literature review, cell-laden injectable hydrogels have been widely applied in bone tissue engineering research. Cell-laden hydrogels have demonstrated excellent injectability, cell viability, and osteogenic properties in both in vivo and in vitro experiments. However, most studies have not analyzed the mechanical properties of the regenerated bone tissue, and the newly-formed bone may not possess satisfactory mechanical performance, limiting the application of injectable hydrogels in weight-bearing bone defects. Moreover, fewer studies have focused on reconstructing a favorable microenvironment with more M2 macrophages and less inflammation, which is also a key factor in promoting bone regeneration. Therefore, the future direction of bone tissue engineering involves the use of novel hydrogel materials combined with biochemical and biomechanical stimuli to ensure that the regenerated bone tissue is well reshaped into natural bone.
References
Van Bael S, Chai YC, Truscello S, Moesen M, Kerckhofs G, Van Oosterwyck H, et al. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012;8:2824–34.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Grambart ST, Anderson DS, Anderson TD. Bone grafting options. Clin Podiatr Med Surg. 2020;37:593–600.
Zhao Y, Cui Z, Liu B, Xiang J, Qiu D, Tian Y, et al. An injectable strong hydrogel for bone reconstruction. Adv Healthc Mater. 2019;8:1900709.
Tang M, Chen W, Weir MD, Thein-Han W, Xu HHK. Human embryonic stem cell encapsulation in alginate microbeads in macroporous calcium phosphate cement for bone tissue engineering. Acta Biomater. 2012;8:3436–45.
Jain M, Matsumura K. Thixotropic injectable hydrogel using a polyampholyte and nanosilicate prepared directly after cryopreservation. Mater Sci Eng C Mater Biol Appl. 2016;69:1273–81.
Alarçin E, Lee TY, Karuthedom S, Mohammadi M, Brennan MA, Lee DH, et al. Injectable shear-thinning hydrogels for delivering osteogenic and angiogenic cells and growth factors. Biomater Sci. 2018;6:1604–15.
Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92:1131–8.
Lippens E, Vertenten G, Gironès J, Declercq H, Saunders J, Luyten J, et al. Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic® F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng Part A. 2009;16:617–27.
Thorpe AA, Freeman C, Farthing P, Callaghan J, Hatton PV, Brook IM, et al. In vivo safety and efficacy testing of a thermally triggered injectable hydrogel scaffold for bone regeneration and augmentation in a rat model. Oncotarget. 2018;9:18277.
Huang Z, Yu B, Feng Q, Li S, Chen Y, Luo L. In situ-forming chitosan/nano-hydroxyapatite/collagen gel for the delivery of bone marrow mesenchymal stem cells. Carbohydr Polym. 2011;85:261–67.
Perez RA, Kim M, Kim TH, Kim JH, Lee JH, Park JH, et al. Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng Part A. 2013;20:103–14.
Watson BM, Vo TN, Tatara AM, Shah SR, Scott DW, Engel PS, et al. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials. 2015;67:286–96.
Vo TN, Shah SR, Lu S, Tatara AM, Lee EJ, Roh TT, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1–11.
Mumcuoglu Guvenc D, Fahmy-Garcia S, Ridwan Y, Nickel J, Farrell E, Kluijtmans S, et al. Injectable BMP-2 delivery system based on collagen-derived microspheres and alginate induced bone formation in a time-and dose-dependent manner. Eur Cell Mater. 2018;35:242–54.
Ingavle GC, Gionet-Gonzales M, Vorwald CE, Bohannon LK, Clark K, Galuppo LD, et al. Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials. 2019;197:119–28.
Sun K, Lin H, Tang Y, Xiang S, Xue J, Yin W, et al. Injectable BMP-2 gene-activated scaffold for the repair of cranial bone defect in mice. Stem Cells Transl Med. 2020;9:1631–42.
Kim HJ, You SJ, Yang DH, Eun J, Park HK, Kim MS, et al. Injectable hydrogels based on MPEG–PCL–RGD and BMSCs for bone tissue engineering. Biomater Sci. 2020;8:4334–45.
Deng L, Liu Y, Yang L, Yi JZ, Deng F, Zhang LM. Injectable and bioactive methylcellulose hydrogel carrying bone mesenchymal stem cells as a filler for critical-size defects with enhanced bone regeneration. Colloids Surf B Biointerfaces. 2020;194:111159.
An C, Liu W, Zhang Y, Pang B, Liu H, Zhang Y, et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomater. 2020;111:181–96.
Tang Y, Lin S, Yin S, Jiang F, Zhou M, Yang G, et al. In situ gas foaming based on magnesium particle degradation: a novel approach to fabricate injectable macroporous hydrogels. Biomaterials. 2020;232:119727.
Wang T, Guo S, Zhang H, Chen Y, Cai Y. Injectable hydrogel delivering bone morphogenetic protein-2, vascular endothelial growth factor, and adipose-derived stem cells for vascularized bone tissue engineering. J Drug Deliv Sci Technol. 2020;57:101637.
Wu J, Li G, Ye T, Lu G, Li R, Deng L, et al. Stem cell-laden injectable hydrogel microspheres for cancellous bone regeneration. Chem Eng J. 2020;393:124715.
Yang J, Liang J, Zhu Y, Hu M, Deng L, Cui W, et al. Fullerol-hydrogel microfluidic spheres for in situ redox regulation of stem cell fate and refractory bone healing. Bioact Mater. 2021;6:4801–15.
Pereira I, Pereira JE, Maltez L, Rodrigues A, Rodrigues C, Oliveira M, et al. Regeneration of critical-sized defects, in a goat model, using a dextrin-based hydrogel associated with granular synthetic bone substitute. Regen Biomater. 2021;8:rbaa036.
Datta S, Rameshbabu AP, Bankoti K, Roy M, Gupta C, Jana S, et al. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater Sci Eng C Mater Biol Appl. 2021;119:111604.
Li D, Yang Z, Zhao X, Luo Y, Ou Y, Kang P, et al. A bone regeneration strategy via dual delivery of demineralized bone matrix powder and hypoxia-pretreated bone marrow stromal cells using an injectable self-healing hydrogel. J Mater Chem B. 2021;9:479–93.
Jiang LB, Ding SL, Ding W, Su DH, Zhang FX, Zhang TW, et al. Injectable sericin based nanocomposite hydrogel for multi-modal imaging-guided immunomodulatory bone regeneration. Chem Eng J. 2021;418:129323.
Shi Z, Zhong Q, Chen Y, Gao J, Pan X, Lian Q, et al. Nanohydroxyapatite, nanosilicate-reinforced injectable, and biomimetic gelatin-methacryloyl hydrogel for bone tissue engineering. Int J Nanomedicine. 2021;16:5603–19.
Liao HT, Tsai MJ, Brahmayya M, Chen JP. Bone regeneration using adipose-derived stem cells in injectable thermo-gelling hydrogel scaffold containing platelet-rich plasma and biphasic calcium phosphate. Int J Mol Sci. 2018;19:2537.
Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30:3371–7.
Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391–7.
Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, et al. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv Funct Mater. 2016;26:2809–19.
Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12:2657–63.
Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25.
Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater. 2012;7:024105.
Parisi-Amon A, Mulyasasmita W, Chung C, Heilshorn SC. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv Healthc Mater. 2013;2:428–32.
Caseiro AR, Ivanova G, Pedrosa SS, Branquinho MV, Georgieva P, Barbosa PP, et al. Human umbilical cord blood plasma as an alternative to animal sera for mesenchymal stromal cells in vitro expansion—A multicomponent metabolomic analysis. PLoS One. 2018;13:e0203936.
Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transpl. 2014;23:505–12.
Song K, Yan X, Li S, Zhang Y, Wang H, Wang L, et al. Preparation and detection of calcium alginate/bone powder hybrid microbeads for in vitro culture of ADSCs. J Microencapsul. 2015;32:811–9.
Varghese S, Hwang NS, Ferran A, Hillel A, Theprungsirikul P, Canver AC, et al. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 2010;28:765–74.
Findlay DM, Haynes DR. Mechanisms of bone loss in rheumatoid arthritis. Mod Rheumatol. 2005;15:232–40.
Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–8.
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85:1544–52.
Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29.
Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10:12–27.
Grellier M, Bordenave L, Amédée J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. 2009;27:562–71.
Acknowledgements
This article was funded by the National Key Research and Development Program of China (2018YFE0104200), the Youth Innovation Promotion Association CAS (2019031) and National Natural Science Foundation of China (51875310, 52175274, 82172065).
Author information
Authors and Affiliations
Contributions
Conceptualization, CXW and YT; Methodology, CXW; Software, CXW; Validation, CXW, SYM and YT; Formal Analysis, CXW; Investigation, CXW; Resources, YT; Data Curation, CXW; Writing—Original Draft Preparation, CXW; Writing—Review & Editing, CXW and YT; Visualization, CXW and YT; Supervision, YT; Project Administration, YT; Funding Acquisition, YT.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Min, S. & Tian, Y. Injectable and Cell-Laden Hydrogel in the Contained Bone Defect Animal Model: A Systematic Review. Tissue Eng Regen Med 20, 829–837 (2023). https://doi.org/10.1007/s13770-023-00569-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00569-2