Abstract
Purpose
Whole-brain radiotherapy (WBRT) may not be beneficial for patients with brain metastases (BMs). The Glasgow Prognostic Score (GPS) is a suggested prognostic factor for malignancies. However, GPS has never been assessed in patients with BMs who have undergone WBRT. The purpose of this study was to determine whether GPS can be used to identify subgroups of patients with BMs who have a poor prognosis, such as recursive partitioning analysis (RPA) Class 2 and Class 3, and who will not receive clinical prognostic benefits from WBRT.
Materials and methods
A total of 180 Japanese patients with BMs were treated with WBRT between May 2008 and October 2015. We examined GPS, age, Karnofsky Performance Status (KPS), RPA, graded prognostic assessment (GPA), number of lesions, tumor size, history of brain surgery, presence of clinical symptoms, and radiation doses.
Results
The overall median survival time (MST) was 6.1 months. seventeen patients (9.4%) were alive more than 2 years after WBRT. In univariate analysis, KPS ≤ 70 (p = 0.0066), GPA class 0–2 (p = 0.0008), > 3 BMs (p = 0.012), > 4 BMs (p = 0.02), patients who received ≥ 3 Gy per fraction (p = 0.0068), GPS ≥ 1 (p = 0.0003), and GPS ≥ 2 (p = 0.0009) were found to significantly decrease the MST. Patients who had brain surgery before WBRT (p = 0.036) had a longer survival. On multivariate analysis, GPS ≥ 1 (p = 0.008) was found to significantly decrease MST.
Conclusion
Our results suggest that GPS ≥ 1 indicates a poor prognosis in patients undergoing WBRT for intermediate and poor prognosis BMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary cause of death from cancer is metastasis [1]. Brain metastases (BMs) are the most common intracranial malignancies and occur in 10–40% of cancer patients [2, 3]. Whole-brain radiation therapy (WBRT) is effective for poor prognosis multiple BMs, defined as being recursive partitioning analysis (RPA) class 2 and class 3 [4, 5]; however, the use of WBRT has recently been decreasing because of improvements in stereotactic radiosurgery (SRS) and other techniques.
The QUARTZ (Quality of Life after Treatment for Brain Metastases) trial recently demonstrated that there was no difference between best supportive care (BSC) alone and BSC plus WBRT in non-small cell lung cancer (NSCLC) patients with poor prognostic factors in quality-adjusted survival years or overall survival (OS) [6]. WBRT for patients with a very poor prognosis may have little clinical utility, and BSC alone may be a more appropriate approach. Therefore, it is necessary to accurately identify patients who are unlikely to benefit from WBRT.
In previous studies, several patient factors were combined with BMs to define patients and groups with specific survival outcome. These prognostic classifications, which are mainly based on age, performance status, and condition of the primary tumor, are represented by RPA and diagnosis-specific graded prognosis assessment (DS-GPA) [4, 5]. However, it has been pointed out that conventional prognostic methods alone are inadequate for predicting short prognoses of BMs.
Recently, several studies have suggested that factors related to systemic inflammation, including the Glasgow Prognostic Score (GPS), are prognostic factors for various malignancies [7,8,9,10]. Although GPS was later modified (mGPS) due to isolated albumin decrements being uncommon, GPS is easy to measure, routinely available, and well standardized worldwide. It has been the subject of various prognostic studies for both operable and inoperable cancers [11]. Despite many patients with cancer being surveyed with results confirming the prognostic utility of GPS in different disease states, this has never been assessed in patients with BMs after WBRT.
The purpose of this study was to determine whether GPS can be used to identify subgroups of BMs that have a poor prognosis such as RPA Class 2 and Class 3, and will not receive clinical prognostic benefits from WBRT.
Materials and methods
Eligibility criteria
This retrospective study was carried out in compliance with the Declaration of Helsinki, and the institutional review board at XXXX approved this study. Between May 2008 and October 2015, we treated 180 Japanese patients with multiple BMs using three-dimensional conformal radiation therapy (3DCRT). We investigated the electronic medical records and radiotherapy records in our institution for patients with BMs. WBRT was performed patients with RPA class 2 or 3 [4]. In this study, patients with lesions that could not be measured were excluded. As a result, patients with only dural metastases or leptomeningeal metastases were excluded. Patients with multiple skull bone metastases were also excluded. Patients who had the diagnosis of the primary tumor confirmed by biopsy or imaging were eligible. Patients with diseases not specified in our institution’s treatment protocols or standard whole-brain irradiation protocols (e.g., ASTRO 2022) were excluded from the study [12]. Specifically, patients with malignant lymphoma, choriocarcinoma, and seminoma were excluded due to differences in irradiation coverage and dosing decisions determined by attending physicians. Figure 1 shows the exclusion criteria in this study.
Flowchart of patients included in the study. There were 260 patients with brain metastases who were treated with whole-brain radiotherapy between 2008 and 2015 in our institution. Twenty-six patients were excluded because they had non-measurable lesions less than 5 mm, dural metastases, bony metastases, and leptomeningeal metastases. Four patients were excluded because they had malignant lymphoma. Additionally, two patients with choriocarcinoma and one patient with seminoma were excluded. Forty-six patients were excluded due to incomplete medical records (e.g., pretreatment blood tests not performed)
Gadolinium-enhanced MRI was performed for each patient before WBRT, while MRI without contrast or CT with contrast was performed in resource-limited settings or for patients with contraindications to MRI. MRI with a 1.5 T MR imaging system included axial T1-weighted spin-echo, axial T2-weighted spin-echo and axial gadolinium-enhanced T1-weighted spin-echo sequences. Sequences such as fluid attenuated inversion recovery (FLAIR) and susceptibility weighted imaging (SWI) were included for some patients. One experienced neuroradiologist and one neuro-radiation oncologist were responsible for image analysis in this study.
CT simulation and whole-brain radiation therapy procedure
All 3DCRT-treated patients were immobilized in the supine position. CT scans were performed at a slice thickness of 2.5 mm with a multidetector CT scanner (GE LightSpeed QX/i; GE Healthcare, Waukesha, WI, USA). The Eclipse™ treatment planning system (Varian Medical Systems, Palo Alto, CA, USA) was used for dose calculations during 3DCRT planning.
The clinical target volume (CTV) included the whole brain. The CTV was expanded in three dimensions with 0.5 cm margins to yield the planning target volume. In this study, a conventional WBRT field was used and hippocampal sparing techniques were not used. Patients were treated with 20–50 Gy using 10 MV photons with a conventional 2–4-field technique. Among the 180 patients included in this study, 106 (58.9%) were treated with 30 Gy/10 fractions, 43 (23.9%) were treated with 36 Gy/12 fractions, 19 (10.6%) were treated with 37.5 Gy/15 fractions, 10 (5.6%) were treated with 20–25 Gy/4–5 fractions, and two (1.1%) were treated with a dose between 40 and 50 Gy.
Glasgow prognostic score evaluations
The GPS was obtained from the levels of CRP and Alb before WBRT and patients were divided into the following three score groups. Patients with both an elevated level of C-reactive protein (> 10 mg/l) and hypoalbuminaemia (< 35 g/l) were allocated to a GPS of 2. Patients in whom only one of those biochemical abnormalities was present were allocated to a group with GPS of 1. Patients in whom neither of those abnormalities was present were allocated to a group with GPS of 0 [7,8,9,10,11].
Outcome assessments and follow-up
OS was defined as the time from the start of WBRT to the date of death from any cause. Follow-up assessments were conducted according to the attending physician's discretion and included blood sampling, CT scans of the trunk, and MRI or CT scans of the head every 1–3 months. These assessments were scheduled every 1–3 months during the first year and every 6 months thereafter.
Statistical analysis
Statistical analyses were performed using JMP v16 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05 in all analyses. The optimal cutoff values for the number of BMs and the maximum diameter of metastases were obtained by calculating the area under the receiver operating characteristic curve (AUROC). The OS rates were determined using Kaplan–Meier estimates. The clinical parameters were investigated using univariate analysis (log-rank test). Hazard ratios with 95% confidence intervals (95% CIs) were calculated by using Cox’s proportional hazard regression model with the following variables: (i) KPS of > 70 at WBRT, (ii) RPA class 2, (iii) GPA ≥ 3, (iv) maximum tumor size ≥ 3.0 cm, (v) number of metastases ≥ 3 or ≥ 4, (vi) with brain surgery (vii) dose per fraction of WBRT, and (viii) GPS ≥ 1.
Results
Patient and brain tumor characteristics
Data for all of the 180 patients were included in the survival analysis. Table 1 shows the characteristics of the patients and the tumors. A total of 1,198 metastases were detected (median: 4.5 metastases per patient).
Survival outcomes
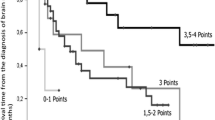
The overall median survival time (MST) was 6.1 months (95% CI: 4.9–8.7 months) (Fig. 2). Patients with GPS 0, GPS 1 and GPS 2 had an MST of 11.1 months (95% CI: 6.7–14.2), 5.1 months (95% CI: 3.3–8.7) and 3.3 months (95% CI: 1.9–5.6), respectively (p = 0.004) (Fig. 3). Notably, 17 patients (9.4%) were alive more than 24 months after WBRT with a median survival period of 36 months (range: 24–37 months). These 17 patients included seven were men and ten were women. The median age of the patients was 59 years (range: 39–78 years). Nine patients were diagnosed with lung cancer, four were diagnosed with breast cancer, two were diagnosed with uterine cancer, one was diagnosed with rectal cancer, and one was diagnosed with cancer of an unknown primary origin. At the time of the initial diagnosis, five patients already had BMs. Among the lung cancers, eight were adenocarcinoma and one was small cell carcinoma. Molecularly targeted drugs were administered in four lung cancer patients. In 16 patients, there were five or fewer BMs. KPS was below 70 in six patients and it was above 70 in eleven patients. According to the RPA classification, there were eleven patients in class 2 and six patients in class 3. GPS was 1 or less in 16 patients, and only one patient had a GPS of 2. The median dose of WBRT was 36 Gy (range: 30–39 Gy). By the time of this prognostic study, eleven patients had passed away. Among the six patients who were still alive, four were in hospice care, one was disease-free, and one was undergoing chemotherapy for recurrent BMs. One patient showed an abscopal effect [13].
Univariate and multivariate outcomes
In univariate analysis, Patients with RPA class 2 had an MST of 8.6 months (95% CI: 5.7–12.2) and patients with RPA class 3 had an MST of 4.7 months (95% CI: 2.7–6.1) (p = 0.019). KPS ≤ 70 at WBRT significantly decreased MST (4.1 [95% CI: 2.5–5.6] vs. 9.0 [95% CI: 6.1–12.2] months, p = 0.0066]). GPA 0–2 significantly decreased MST (5.0 [95% CI: 3.4–7.2] vs. 10.9 [95% CI: 5.7–13.9] months, p = 0.0008]). Having > 3 BMs, versus ≤ 3 BMs, decreased the survival period (5.3 [95% CI: 3.8–7.4] vs. 9.8 [95% CI 5.7–13.5] months, p = 0.012). Having > 4 BMs, versus ≤ 4 BMs, decreased the survival period (5.2 [95% CI: 3.6–7.4] vs. 8.4 [95% CI 5.7–11.7] months, p = 0.022).
A significant difference was found in MST between patients with and those without brain surgery before WBRT (12.2 [95% CI: 5.7–88.6] vs. 5.6 [95% CI: 4.4–8.5] months, p = 0.036). Although the total dose of WBRT (≥ 30 vs. < 30 Gy) was not a significant prognostic factor (p = 0.15), patients who received ≥ 3 Gy per fraction died sooner than did patients who received < 3 Gy per fraction (5.6 [95% CI: 4.2–6.9] vs.14.2 [95% CI: 5.6–27.6] months, p = 0.0068]. Furthermore, GPS ≥ 1 was significantly associated with decreased MST (4.2 [95% CI: 2.5–5.7] vs.9.8 [95% CI: 6.2–13.1] months, p = 0.0005).
Univariate analysis revealed that none of the following variables were significant prognostic factors: primary tumor site, histological type, presence of ascites or pleural effusion, steroid medications, surgery, and targeted therapy. Multivariate analysis showed that GPS ≥ 1 was significantly indicated poor prognosis. These results are shown in Table 2.
Discussion
Glasgow Prognostic Score (GPS) is an index that combines serum CRP and serum Alb levels, and it is a known prognostic factor for nonsmall cell lung cancer [11]. GPS has also been shown to provide additional prognostic information for patients with advanced cancer in various organs [7,8,9,10]. Previous studies have revealed that low serum Alb levels signify poor prognosis in patients with BMs [14, 15]. Therefore, it is reasonable to consider GPS as an important clinical prognostic factor in patients with BMs.
GPS has not been reported as a prognostic factor after WBRT for BMs. Patients with GPS ≥ 1 had a significantly shorter survival period than did patients with GPS < 1 in this study. WBRT is a form of palliative therapy, and patients undergoing WBRT are at high risk of high CRP and low Alb levels. Patients with BMs are also at risk for systemic inflammation due to prolonged use of chemotherapy and systemic metastases. Hence, it may be beneficial to evaluate GPS as a prognostic factor before WBRT. It is not unexpected that GPS serves as an indicator of overall physical status and exerts an influence on OS. However, from a clinical perspective, for example, it could be beneficial in patients when assessment of RPA or DS-GPA becomes challenging. It is notable that the majority of the patients with long-term survival had a GPS of 1 or less.
According to the QUARTZ trial, WBRT for patients with brain metastases from NSCLC provided no additional clinically significant benefit [6]. The present study included patients with a relatively poor prognoses, and the GPS ≥ 1 patient group might have included patients for whom WBRT can be avoided.
Some extracranial tumor factors are associated with the prognosis for patients with BMs. One significant prognostic factor is primary tumor control [4, 24]. In our study, the primary tumor was controlled in 11 (5.6%) of the 180 patients, a percentage that is similar to that in another study [17]. Gaspar et al. reported that the median survival periods after WBRT were 7.1, 4.2, and 2.3 months for patients with RPA class 1, class 2, and class 3, respectively [4]. Although recent advances in treatment techniques have improved outcomes for patients in each class, the prognosis generally remains poor [18]. In our study, the OS periods for RPA class 2 and class 3 patients were 8.6 months (95% CI: 5.7–12.2) and 4.7 months (95% CI: 2.7–6.1), respectively (p = 0.019). OS periods tended to be similar to or slightly longer than those previously reported. In this study, histology and the presence of ascites or pleural effusion were not found to be significant prognostic factors; however, the reasons for this were not clear. Given that this study focused on the poor prognosis group, it is plausible that a large number of patients were in the advanced stages of their disease, for which the impact of histological type might be less prominent. While pleural fluid and ascites did not emerge as prognostic factors, it is notable that GPS might be influenced by the inclusion of albumin levels, which are connected to colloid osmotic pressure [19]. However, we acknowledge that delving into this topic within this study might be challenging.
KPS, RPA, and GPA were shown to be significant prognostic factors in our univariate analysis, as was found in studies by the Radiation Therapy Oncology Group (RTOG) [4, 24]. The fact that these factors did not show significant differences in our multivariate analysis may be due to the inclusion of many poor prognosis BMs in the present study [20].
DS-GPA has been proposed for lung cancer, breast cancer, gastrointestinal cancer, renal cancer, and malignant melanoma [5]. In recent years, the prognosis of lung cancer has depended on the presence or absence of EGFR mutation and ALK fusion protein [21]. In our study, 121 patients (67.8%) had lung cancer, and those patients included 82 patients (42.5%) with lung adenocarcinoma. Targeted therapy was performed in 29 patients (16.1%) but it was not a clear prognostic factor in our univariate analysis.
Various radiation doses including 20 Gy/4–5 fractions, 30 Gy/15 fractions, 30 Gy/10 fractions, 37.5 Gy/15 fractions, and 40 Gy/20 fractions are used for WBRT [22]. Although we found no significant difference in survival between the various total doses in our study, a dose of 40 Gy/20 fractions was found to be significantly superior over 20 Gy/4–5 fractions for intracranial tumor control in two randomized controlled trials [23,24,25]. Good prognosis has been reported in patients who received WBRT at a dose of 2–2.5 Gy for breast cancer [26]. The present study included 26 breast cancer patients (14.4%), and that may be the reason for the significant difference in univariate analysis. Concerning dose fractionation for WBRT, it is important to discuss how to select the dose fraction, and selection bias might therefore be one of the causes of the difference in prognosis in this study.
This study had some limitations. Since this study was retrospective and conducted only for patients in whom WBRT was performed, the possibility of bias in the selection of patients for analysis of the OS rate cannot be ruled out. Moreover, since only OS was evaluated in our study, it might represent the prognosis of patients eligible for WBRT rather than the efficacy of WBRT. In multivariate analysis, only GPS (≥ 1 vs. < 1) showed a significant difference in MST; however, we could not find a clear reason for there being a significant difference with GPS only. This study included relatively young patients. However, it was not possible to analyze the prognostic impact of these younger patients. All the patients predate the use of standardized criteria such as response assessment in neuro-oncology brain metastases criteria, and tumor response was difficult to assess and progression-free survival was not assessed, which was an important drawback. The total doses varied and some patients included doses as high as 40–50 Gy, which are not used these days. Additionally, a common complication following WBRT is neurocognitive function (NCF) impairment, which can decrease the quality of life for patients after WBRT [24]. Many of the patients in this study were elderly, and NCF was not assessed before treatment. Therefore, cognitive function after WBRT, which is a clinically important indicator, could not be analyzed.
Conclusion
The aim of this study was to determine whether GPS can be used to identify subgroups of patients with BMs who have intermediate and poor prognoses and would not benefit from WBRT to improve prognosis. Our findings suggest that a GPS score of ≥ 1 is associated with a poor prognosis in patients undergoing WBRT. WBRT does not improve prognosis beyond its palliative effects in patients with a GPS score of 1 or higher, and treatment with BSC alone may be considered an option for patients with poor prognosis. Prospective research is needed to confirm the results of this study. Furthermore, investigating the relationship between GPS and the prognosis of WBRT for different diseases, such as lung and breast cancers, will be necessary in the future.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Alb:
-
Albumin
- AUROC:
-
Area under the receiver operating characteristic curve
- BMs:
-
Brain metastases
- CTV:
-
Clinical target volume
- CT:
-
Computed tomography
- DS-GPA:
-
Diagnosis-specific graded prognosis assessment
- FLAIR:
-
Fluid Attenuated Inversion Recovery
- GPS:
-
Glasgow Prognostic Score
- HMs:
-
Hippocampal metastases
- KPS:
-
Karnofsky performance status
- MRI:
-
Magnetic resonance imaging
- mGPS:
-
Modified Glasgow Prognostic Score
- NCF:
-
Neurocognitive function
- OS:
-
Overall survival
- RPA:
-
Recursive partitioning analysis
- RTOG:
-
Radiation Therapy Oncology Group
- SRS:
-
Stereotactic radiosurgery
- SWI:
-
Susceptibility weighted imaging
- WBRT:
-
Whole-brain radiotherapy
- 3DCRT:
-
Three-dimensional conformal radiation therapy
References
Fidler IJ (2002) Critical determinants of metastasis. Semin Cancer Biol 12:89–96. https://doi.org/10.1006/scbi.2001.0416
Nussbaum ES, Djalilian HR, Cho KH, Hall WA (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78:1781–1788
Khuntia D, Brown P, Li J, Mehta MP (2006) Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol Off J Am Soc Clin Oncol 24:1295–1304. https://doi.org/10.1200/JCO.2005.04.6185
Gaspar L, Scott C, Rotman M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751. https://doi.org/10.1016/s0360-3016(96)00619-0
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol Off J Am Soc Clin Oncol 30:419–425. https://doi.org/10.1200/JCO.2011.38.0527
Agarwal JP, Chakraborty S, Laskar SG et al (2018) Applying the QUARTZ trial results in clinical practice: development of a prognostic model predicting poor outcomes for non-small cell lung cancers with brain metastases. Clin Oncol (R Coll Radiol) 30:382–390. https://doi.org/10.1016/j.clon.2018.02.002
Forrest LM, McMillan DC, McArdle CS et al (2003) Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 89:1028–1030. https://doi.org/10.1038/sj.bjc.6601242
Topkan E, Selek U, Ozdemir Y et al (2018) Prognostic value of the glasgow prognostic score for glioblastoma multiforme patients treated with radiotherapy and temozolomide. J Neurooncol 139:411–419. https://doi.org/10.1007/s11060-018-2879-4
Golder AM, McMillan DC, Park JH et al (2021) The prognostic value of combined measures of the systemic inflammatory response in patients with colon cancer: an analysis of 1700 patients. Br J Cancer 124:1828–1835. https://doi.org/10.1038/s41416-021-01308-x
Zhuang Z, Li Y, Hong Y et al (2022) A novel prognostic score based on systemic inflammatory biomarkers for patients with oral squamous cell carcinoma. Oral Dis 28:631–638. https://doi.org/10.1111/odi.13774
Mcmillan DC (2013) Cancer treatme nt revie ws the systemic inflammation-based glasgow prognostic score : a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540. https://doi.org/10.1016/j.ctrv.2012.08.003
Vogelbaum MA, Brown PD, Messersmith H et al (2022) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol Off J Am Soc Clin Oncol 40:492–516. https://doi.org/10.1200/JCO.21.02314
Ishikawa Y, Umezawa R, Yamamoto T et al (2022) Differential abscopal effect in extracranial and intracranial lesions after radiotherapy alone for vertebral bone metastasis of unknown primary: a case report. J Med Case Rep 16:94. https://doi.org/10.1186/s13256-022-03321-x
Nieder C, Marienhagen K, Dalhaug A et al (2014) Prognostic models predicting survival of patients with brain metastases: integration of lactate dehydrogenase, albumin and extracranial organ involvement. Clin Oncol (R Coll Radiol) 26:447–452. https://doi.org/10.1016/j.clon.2014.03.006
Nieder C, Norum J, Dalhaug A et al (2013) Best supportive care in patients with brain metastases and adverse prognostic factors: development of improved decision aids. Support care cancer Off J Multinatl Assoc Support Care Cancer 21:2671–2678. https://doi.org/10.1007/s00520-013-1840-5
Gaspar LE, Scott C, Murray K, Curran W (2000) Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 47:1001–1006. https://doi.org/10.1016/s0360-3016(00)00547-2
Enders F, Geisenberger C, Jungk C et al (2016) Prognostic factors and long-term survival in surgically treated brain metastases from non-small cell lung cancer. Clin Neurol Neurosurg 142:72–80. https://doi.org/10.1016/j.clineuro.2016.01.011
Nieder C, Mehta MP (2009) Prognostic indices for brain metastases–usefulness and challenges. Radiat Oncol 4:10. https://doi.org/10.1186/1748-717X-4-10
Planas R, Montoliu S, Ballesté B et al (2006) Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 4:1385–1394. https://doi.org/10.1016/j.cgh.2006.08.007
Miyazawa K, Shikama N, Okazaki S et al (2018) Predicting prognosis of short survival time after palliative whole-brain radiotherapy. J Radiat Res 59:43–49. https://doi.org/10.1093/jrr/rrx058
Sperduto PW, Yang TJ, Beal K et al (2017) Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 3:827–831. https://doi.org/10.1001/jamaoncol.2016.3834
Batumalai V, Descallar J, Delaney GP et al (2021) Patterns of palliative radiotherapy fractionation for brain metastases patients in New South Wales, Australia. Radiother Oncol 156:174–180. https://doi.org/10.1016/j.radonc.2020.12.020
Davey P, Hoegler D, Ennis M, Smith J (2008) A phase III study of accelerated versus conventional hypofractionated whole brain irradiation in patients of good performance status with brain metastases not suitable for surgical excision. Radiother Oncol J Eur Soc Ther Radiol Oncol 88:173–176. https://doi.org/10.1016/j.radonc.2008.05.020
Graham PH, Bucci J, Browne L (2010) Randomized comparison of whole brain radiotherapy, 20 Gy in four daily fractions versus 40 Gy in 20 twice-daily fractions, for brain metastases. Int J Radiat Oncol Biol Phys 77:648–654. https://doi.org/10.1016/j.ijrobp.2009.05.032
Murray KJ, Scott C, Greenberg HM et al (1997) A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the radiation therapy oncology group (RTOG) 9104. Int J Radiat Oncol Biol Phys 39:571–574. https://doi.org/10.1016/s0360-3016(97)00341-6
Okada Y, Kobayashi M, Shinozaki M et al (2020) Survival time and prognostic factors after whole-brain radiotherapy of brain metastases from of breast cancer. Acta Radiol open 9:2058460120938744. https://doi.org/10.1177/2058460120938744
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishikawa, Y., Umezawa, R., Yamamoto, T. et al. Glasgow prognostic score for assessing the efficacy of whole-brain radiation therapy in cases of recursive partitioning analysis class 2 and class 3 multiple brain metastases: a retrospective study. Acta Neurol Belg 124, 231–239 (2024). https://doi.org/10.1007/s13760-023-02384-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02384-x