Abstract
Introduction
Acute necrotizing encephalopathy (ANEC) is a rare entity characterized by encephalopathy following a febrile illness. Most patients are sporadic; however, recurrent and familial cases have been associated with RAN-binding protein 2 (RANBP2) mutation. Well-defined MRI findings can even be life-saving with early diagnosis and treatment.
Methods
In this article, nine pediatric cases diagnosed with ANEC1 both clinically and radiologically, and with least one variation in the RANBP2 gene, are presented.
Results
All patients were previously healthy and presented with encephalopathy after an acute febrile infection. The patients of 44% had a similar attack history in their family. Influenza A/B was detected in 7 patients (78%). One patient was admitted at age 32 years old. The first clinical findings of patients were encephalopathy (100%), seizure (44%), vision problems (33%), ataxia (11%), and monoplegia (11%). Recurrent attacks were seen in two (22%) patients. Brain MRI findings including bilateral thalamus, external capsules, and brainstem involvements were highly suggestive for RANBP2 mutation. Based on MRI findings, genetic analyses were quickly performed and confirmed. All of the patients were treated with empirical encephalitis treatment, oseltamivir, intravenous immunoglobulin (IVIG), high-dose steroid and, if necessary, plasmapheresis, but three (33%) patients died despite treatment.
Conclusion
ANEC associated with RANBP2 mutation may occur early or late-onset and can be recurrent and fatal. Therefore, early diagnosis and treatment have the potential to modify the severity of this encephalopathy. Well-defined MRI findings are highly instructive for early diagnosis.
Similar content being viewed by others
Introduction
Acute necrotizing encephalopathy of childhood (ANEC) is a rapidly progressing encephalopathy that develops after acute infections in children between 6 and 18 years old [1]. It presents with fever, deteriorating consciousness, personality changes, seizures, focal deficits, and coma [2]. The disease is mostly associated with preceding viral infection, including influenza A/B, parainfluenza, human herpesvirus 6, enterovirus, and varicella. However, it rarely occurs due to bacterial infections, such as Mycoplasma pneumoniae, diphtheria, and tetanus. Recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019) (COVID-19)-related ANEC cases have also been reported [3]. Inflammatory cytokine storm, which is mediated by Interleukin 6 (IL-6), Interleukin 8 (IL-8), and Tumor Necrosis Factor Alpha (TNF-α), has been hypothesized in the pathogenesis of ANEC [4,5,6]. Hyper-intense signal changes are observed in symmetrical bilateral thalamus and brain stem, particularly in T2-weighted and fluid-attenuated inversion recovery (FLAIR) weighted magnetic resonance imaging (MRI) [6,7,8]. Diffusion restrictions that develop secondary to cytotoxic and vasogenic edema in diffusion-weighted series are also helpful in early diagnosis [8, 9].
Most cases of ANEC are sporadic and nonrecurrent. However, RAN-binding protein 2 (RANBP2)-associated type (ANEC1) is the rarer, familial, recurrent and genetic type. In 2003, 11 individuals from the same family were diagnosed with ANEC by Neilson et al. [10]. It was stated that this disease could be inherited as autosomal dominant. In 2009, the RANBP2 gene mutation was defined for the first time in 10 unrelated patients diagnosed with ANEC [4]. RANBP2 is a nuclear protein produced in all tissues, with an extensive intracellular function [4]. ANEC1 inherited as autosomal dominant (OD) shows incomplete penetrance. Therefore, the probability of ANEC1 development in a person who has RANBP2 mutation is 40% in their lifetime [4, 10, 11]. It should be noted that these patients have relatives who were followed up with diagnoses, such as acute disseminated encephalomyelitis (ADEM), Leigh's disease, encephalitis, or aseptic meningitis. RANBP2 gene has many functions, including mitochondrial, metabolic and nuclear signals, and its mutations lead to different clinical manifestations depending on the underlying mutation type. If mutation disrupts nuclear signal function, impairment in the blood–brain barrier may be seen. Additionally, RANBP2 mutation leading to mitochondrial dysfunction can cause cell death secondary to energy depletion. Thus, the involvement pattern of the disease resembles the energy depletion diseases, such as Wernicke's encephalopathy and Leigh syndrome [12, 13]. In this article, nine pediatric cases diagnosed with ANEC1 both clinically and radiologically, and with at least one variation in the RANBP2 gene are presented.
Materials and methods
This is a retrospective study of patients diagnosed with ANEC1 in four tertiary centers in Turkey from 2016 to 2020. These centers were Istanbul Medipol University Faculty of Medicine, Karadeniz Technical University Faculty of Medicine, Republic of Turkey, Ministry of Health Bursa Provincial Health Directorate, University of Health Sciences, Ministry of Health University Adana City Training and Research Hospital. Ethics committee approval was obtained from the Adana City Training and Research Hospital with the decision dated 27/01/2021 and numbered 1301.
Nine ANEC1 patients with RANBP2 gene variants were included in our study. All neurological examinations of the patients were recorded. Biochemical studies of plasma amino acids, acylcarnitine profile, urinary organic acids, and lactate were performed. Brain magnetic resonance imaging (MRI) was obtained. Diffusion MRI study was the initial technique due to no need for sedation and was later followed by a full sequences study.
Results
The median age of the pediatric patients was 5 years (min: 0.75 max: 10 years); there was one 32-year-old patient. Four of them were female (44%), and 5 of them were male (55%). Four of the patients (44%) had a similar attack history in their family. Seven of them (77%) had their first attack in the winter season. They all had a preceding infection history when the ANEC1 clinical findings occurred, and influenza A/B was detected in 77% of them. All of the patients presented with encephalopathy, four of them (44%) with seizures, three of them (33%) with sudden vision problems, one of them (11%) with monoplegia, and one of them (11%) with ataxia (Table 1). First, diffusion MRI was performed in all patients. Bilateral thalamus involvement was seen on diffusion and brain MRI in all patients, and other areas of involvement, including the external capsule, basal ganglia and cerebellar region, are summarized in Table 2. Spinal cord involvement was seen in 2 patients (22%). The liver function test results were normal in 7 patients (77%), and basal metabolic tests and cerebrospinal fluid (CSF) culture test results were normal. All patients were treated with empirical encephalitis treatment (ceftriaxone, clarithromycin, and acyclovir), oseltamivir, pulse steroid, and intravenous immunoglobulin (IVIG) for 5 days after diffusion MRI was performed. Treatment was continued with 2 mg/kg methylprednisolone for eight weeks. Four patients had plasmapheresis if response to initial therapies was inadequate. One patient (11%) had 3 attacks; 1 patient (11%) had 2 attacks; and 4 patients (44%) were intubated. The 32-year-old mother of the patient who had 3 attacks was also diagnosed with ANEC1 and died. Three patients (33%) improved completely, 3 patients had (33%) paraparesis and 3 patients (33%) died. Two of the patients died at the first attack, one of the patients died at the third attack. At least one pathogenic variant in the RANBP2 gene was found in all patients (Table 3). Detailed demographic, clinical, and laboratory data of the patients are summarized in the tables.
Patient 1
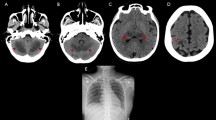
Eight-year-old male presented with sudden unsteady walking, headache and encephalopathy after a fever and upper respiratory tract infection (URTI) (Table 1). There was a similar attack history among the patient's family. On the first day of admission, MRI showed diffusion restrictions and T2-weighted abnormalities in the thalamus, periventricular white matter, mammillary body, external capsule, corpus callosum splenium, basal ganglia, and temporal regions bilaterally (Figs. 1a–c and 2a and b). Empirical encephalitis treatment, pulse methylprednisolone, and IVIG treatments for five days were followed by plasmapheresis (Table 3). Maintenance treatment was continued with 2 mg/kg methylprednisolone for eight weeks. After one year, repeat MRI was normal, the gross motor function classification system score (GMFCS) was one, he had no sequelae and further attacks.
Diffusion MRI of the patients. Patient 1: a–c, patient 2: d–e, patient 3: f–g, patient 4: h. All diffusion MRIs of the patients were taken on the first day of the illness. From a to c, images show extensive involvement to the basal ganglia, mammillary body, splenium of the corpus callosum, and temporal lobes. D and E images show bilateral thalamus involvement. F and G images show a necrotizing diffusion restriction in lentiform nuclei. H image shows involvement in the bilateral thalamus and pons
Brain MRI of the patients. a and b show the flair and coronal T2-weighted images of patient 1. There is extensive involvement in the bilateral thalamus, basal ganglia, mammillary body, and splenium of the corpus callosum. c and d show the flair and T2-weighted images of patient 2, and it was taken after the first month of the illness. There are some atrophy and nonspecific gliotic lesion. e and f show the flair and T2-weighted images of patient 3. There is a necrotizing lesion in the lentiform nuclei. g and h show the flair and T2-weighted images of the patient 4. There is extensive involvement in the bilateral thalamus, mammillary body, basal ganglia, and pons
Patient 2
A five-year-old male was admitted with vomiting, seizures and encephalopathy after fever and URTI for a week. After the seizure, he rapidly became unconscious and comatose, and was intubated (Table 1). Diffusion MRI performed on the first day of admission showed diffusion restrictions in the bilateral thalamus, temporal lobe and pons. On the first day of admission, empirical encephalitis treatment, pulse steroid, and IVIG were started followed by plasmapheresis and, then, tapering of oral methylprednisolone. Repeat MRI one month later showed atrophy and a nonspecific gliotic lesion (Figs. 1d, e and 2c, d). He had dystonia on discharge with GMFCS of 3. After 1 year, he had no further attacks and an exam with GMFCS of 1 and no dystonia (Table 3).
Patient 3
Nine-month-old boy presented with acute right arm monoplegia and encephalopathy after fever and URTI (Table 1). Diffusion restriction and necrotizing involvement in the left lentiform nucleus were seen on MRI (Figs. 1f, g and 2e, f). Encephalitis treatment, pulse steroid, and IVIG for five days were started (Table 3). Repeat MRI showed a lesion on the basal ganglia. The patient could raise his right arm to head level with 4/5 muscle strength one year later.
Patient 4
A fourteen-month-old female presented with encephalopathy and vomiting for three days after fever and diarrhea for a week. There was a similar attack history in the patient's family (Table 1). The patient was examined three days after the onset of symptoms, and treatment was started on the third day. MRI was abnormal in the bilateral external capsule, thalamus, and pons (Figs. 1h and 2g, h). Initial therapy was broad-spectrum antibiotics, acyclovir, high-dose glucocorticoid and IVIG followed by plasmapheresis (Table 3) and, then, methylprednisolone 2 mg/kg/day for eight weeks. Repeat MRI was normal. She had no new attacks on 1-year follow-up. The exam showed diffuse dystonia and GMFCS of five.
Patient 5
She had three attacks. During the first attack, she presented with seizures and encephalopathy after fever and URTI. Diffusion restrictions and signal increases without contrast enhancement in the bilateral thalamus, pons, medulla oblongata, and cerebellum were seen on the diffusion and T2-weighted MRI (Fig. 3). Empirical antibiotics, acyclovir, high-dose steroid, and IVIG were started for presumed diagnosis of ADEM and encephalitis. Exam at discharge found GMFCS of four, paraparesis and difficulty in swallowing (Table 3). Six months later, she had a second attack presenting with fever, loss of consciousness and seizures (Table 1) treated with high-dose steroids, IVIG and antibiotics. MRI showed signal increases in the bilateral thalamus, hippocampus, periaqueductal area, and pons (Fig. 4). Exam was abnormal at discharge (Table 3). On the third attack one year later, she presented with fever, URTI and decreased consciousness. There was a high signal intensity in the thalamus, cerebellum and cervical spinal cord on MRI (Fig. 5). She was treated with antibiotics, high-dose steroids, and IVIG, but died during her third hospitalization (Table 3). During this time, her mother was hospitalized due to blurred vision, encephalopathy, and seizures after similar fever and URTI symptoms. MRI showed diffusion restrictions and signal increases in the bilateral thalamus, capsule externa and basal ganglia (Fig. 6). She was treated with broad-spectrum antibiotics, acyclovir, IVIG, and high-dose steroid, but died. RANBP2 gene mutation testing was not done (Table 3).
Patient 6
He was hospitalized with encephalopathy and coma after URTI and fever. He had two attacks at the age of two and six months old (Table 1). MRI showed diffusion restrictions and T2 signal increases in the bilateral thalamus, external capsule, mesencephalon, pons, and mammillary body (Fig. 7). Antibiotics, high-dose steroids, and IVIG were started. GMFCS was three in follow-up. No new attack occurred (Table 3).
Patient 7
Five-year-old girl presented with loss of consciousness and seizure after fever and URTI (Table 1). After the seizure, she rapidly became unconscious and comatose and was intubated. MRI showed diffusion restrictions and signal increases in the bilateral thalamus, mesencephalon, pons, external capsule, mammillary body, and temporal lobes (Fig. 8). Encephalitis treatment, IVIG, and high-dose steroid were started, followed by a tapered dose of prednisolone for eight weeks (Table 3), but she died.
Patient 8
Ten-year-old girl presented with sudden vision loss after a fever (Table 1). Bilateral thalamus, external capsule, and pons signal increases and diffusion restrictions were seen on MRI (Fig. 9). She was treated with encephalitis treatment, IVIG, and high-dose steroid followed by a tapered dose of deltacortil (Table 3). Follow-up MRI showed abnormal signal in the left external capsule. She had no further attacks and GMFCS of three.
Patient 9
Ten-year-old boy presented with seizures and blurred vision after fever and URTI (Table 1). He received encephalitis treatment, IVIG and high-dose steroid followed by deltacortil. His general status gradually deteriorated so was intubated and treated with plasmapheresis. On MRI, there was a signal increase and diffusion restriction in the bilateral thalamus, external capsule, mesencephalon, pons, and temporal lobe (Fig. 10). Increased signal intensity and diffuse bleeding were shown on spinal MRI. The patient died (Table 3).
Discussion
ANEC1 is an extremely rare disease that requires rapid diagnosis and treatment. Experiences with therapeutic interventions and follow-up of patients with ANEC1 are limited and usually consist of case series. Early treatment with steroids might be correlated with a favorable prognosis, presumably secondary to interrupting elevated cytokines and decreasing inflammation [14]. High-dose steroids can also limit the edema associated with neuroinflammation [13]. The outcome of ANEC1 varies from complete recovery to persistent sequelae or death. The mortality rate of ANEC1 is 30%.
Studies have reported that the prognosis varies according to the duration and number of seizures, the time of diagnosis and treatment initiation, the number of recurrences and the areas of involvement in the brain [15, 16]. Sell et al. [17] reported 2 cases with poor prognosis who had seizures, more than one attack and typical involvement pattern on MRI. Lee et al. [14] also reported that two patients had seizures and a fatal prognosis despite aggressive immunosuppressive therapy and IVIG on the first day. In our study, we found that the prognosis of cases who had more than one attack, (patient 5 and patient 6) were intubated after seizures (patient 7 and patient 9), or started treatment late (patient 4), was quite poor. The only exception was the second case. He was admitted with a seizure and was intubated, but he could walk independently after one year, possibly due to the fact that he had a single attack and was treated with plasmapheresis and pulse steroid followed by prednisolone taper. Patients 9 and patient 5 died, suggesting that spinal involvement may be also associated with a poor prognosis.
Cases with more than 2 attacks have not been reported in the literature. Patient 5 had three attacks and died during the third attack. Also, her mother had an attack and died. On the other hand, presentation with acute hemiplegia and acute visual loss is infrequent in the literature. Sell et al. [17] reported a patient presented with encephalopathy and acute hemiplegia, and Chew et al. [18] reported a patient whose first complaint was acute vision loss. Three of our patients presented with acute vision loss, likely related to involvement of the bilateral thalamus and lateral geniculate nucleus.
For our population, diffusion MRI was critical in the evaluation of ANE or ANEC1 because it was not easy to perform conventional MRI due to the technical and patient challenges. Albayram et al. [19] also reported that diffusion MRI could be performed in ANE cases and that diffusion restrictions might be observed due to vasogenic edema and necrosis. In our study, diffusion MRI was performed after emergency department presentation since conventional MRI would be technically challenging (need for anesthesia). When the typical involvement areas were observed (bilateral thalamus, external capsule, cerebellum, and basal ganglia), steroid and IVIG treatments were started quickly.
As previously reported, 100% of the patients had bilateral thalamus, 89% of them had external capsule, and 78% of them had brain stem and mesencephalon involvement. Patient 1 had corpus callosum involvement, which was not previously reported. Levine et al. [3] and Okumara et al. [15] reported that oseltamivir, IVIG, and plasmapheresis were ineffective in ANEC1 patients. However, we had favorable results in our patients who received pulse steroid, IVIG, and plasmapheresis early in the course of their illness. In our study, we performed plasmapheresis for patients 1, 2, 4, and 9. Only patient 9, who started plasmapheresis after clinical deterioration, died. Plasmapheresis might be helpful because of possible immune dysregulation due to genetic mutation.
The coexistence of ANEC and influenza is common. Therefore, influenza vaccines are recommended for patients with a history of ANEC and their relatives for further infections and possible recurrences [20, 21]. In our study, we suggested that patients should be monitored closely during the influenza season, and encouraged annual influenza vaccination.
The function of the RANBP2 gene is still unclear and clinical difference in patients may be due to the differences in mutations. Very few cases have been reported in the literature. The RANBP2 gene (601,181) is localized on chromosome 2q12. The missense p.Thr585Met mutation is most frequently observed. The RANBP2 gene is both Ran GTPase-dependent and -independent. Ran GTPase-dependent RANBP2 gene is responsible for the nuclear and pro-inflammatory function, whereas Ran GTPase-independent RANBP2 gene is responsible for mitochondrial function. Depending on the gene mutation, cell destruction, cytokine dysregulation, blood–brain barrier destruction, oxidative stress, and energy metabolism disorder can be seen [18]. Ohashi et al. [22] reported a pediatric case with concomitant RANBP2 and carnitine palmitoyltransferase 2 (CPT2) gene mutation, and stated that CPT2 gene mutation might also cause the onset of acute encephalopathy. Shibata et al. reported that the mutated RANBP2 gene had an attenuated binding ability to COX11. Therefore, this change might lead to a decrease in ATP production and energy deficiency, followed by the onset of encephalopathy [23]. Thus, reporting of all mutations is important for clarifying the function and clinic heterogeneity of the gene.
Conclusion
ANEC associated with RANBP2 gene mutation may occur early or late-onset and can be recurrent and fatal. If a previously healthy person has seizures, encephalopathy or sudden loss of consciousness after fever and URTI, and specific region involvement on brain MRI or diffusion MRI, the RANBP2 gene is indicated. We also recommend high-dose steroids and IVIG; if there is no clinical improvement, plasmapheresis can be performed. Influenza vaccination and close monitoring during influenza season can reduce the risk of possible recurrence. Early diagnosis, including brain MRI with characteristic findings, and treatment, have the potential to modify the severity of this encephalopathy.
Limitations
This was the retrospective and multicenter study. The number of patients with ANEC1 is not enough, so the experiences of therapeutic interventions and follow-up of patients with ANEC1 are limited and usually consist of case series. As more cases are gathered and multicenter studies are designed, we will conduct more detailed analyses and more comprehensive follow-ups.
Abbreviations
- ANEC:
-
Acute necrotizing encephalopathy of childhood
- OD:
-
Autosomal dominant
- CSF:
-
Cerebrospinal fluid
- FLAIR:
-
Fluid attenuated inversion recovery
- GMFCS:
-
Gross motor function classification system
- MRA:
-
Magnetic Resonance Angio
- RANBP2 :
-
RAN-binding protein 2
- SWI:
-
Susceptibility Weighted Imaging sequence
- URTI:
-
Upper respiratory tract infection
References
Mizuguchi M (1997) Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 19:81–92. https://doi.org/10.1016/S0387-7604(96)00063-0
Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M (2007) Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl 186:45–56. https://doi.org/10.1111/j.1600-0404.2007.00809.x
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 296:119–120. https://doi.org/10.1148/radiol.2020201187
Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, Kerr DS (2009) Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 84:44–51. https://doi.org/10.1016/j.ajhg.2008.12.009
Narita M (2002) Acute necrotizing encephalopathy by Mycoplasma pneumoniae infection? Arch Intern Med 162:1647. https://doi.org/10.1001/archinte.162.14.1647-a
Neilson DE (2010) The interplay of infection and genetics in acute necrotizing encephalopathy. Curr Opin Pediatr 22:751–757. https://doi.org/10.1097/MOP.0b013e3283402bfe
Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL (2015) Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm. https://doi.org/10.1155/2015/792578
Carmo RLD, Alves Simao AK, Amaral LLFD, Inada BSY, Silveira CF, Campos CMS (2019) Neuroimaging of emergent and reemergent infections. Radiographics 39:1649–1671. https://doi.org/10.1148/rg.2019190020
Sener RN (2005) Acute necrotizing encephalopathy. Eur Radiol 15:395–396. https://doi.org/10.1007/s00330-004-2395-0
Neilson DE, Eiben RM, Waniewski S, Hoppel CL, Varnes ME, Bangert BA et al (2003) Autosomal dominant acute necrotizing encephalopathy. Neurology 61:226–230. https://doi.org/10.1212/01.WNL.0000073544.28775.1A
Neilson DE (2014) Susceptibility to Infection-Induced Acute Encephalopathy 3-RETIRED CHAPTER, FOR HISTORICAL REFERENCE. GeneReview. www.ncbi.nlm.nih.gov/books/NBK258641
Singh RR, Sedani S, Lim M, Wassmer E, Absoudet M (2015) RanBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur J Paediatr Neurol 19:106–113. https://doi.org/10.1016/j.ejpn.2014.11.010
Levine JM, Ahsan N, Ho E, Santoro JD (2020) Genetic acute necrotizing encephalopathy associated with RANBP2: Clinical and therapeutic implications in pediatrics. Multiple Sclerosis and Related Disorders 43:102194. https://doi.org/10.1016/j.msard.2020.102194
Lee YJ, Hwang SK, Lee SM, Kwon S (2017) Familial acute necrotizing encephalopathy with RANBP2 mutation: the first report in Northeast Asia. Brain Develop 39:625–628. https://doi.org/10.1016/j.braindev.2017.02.005
Okumura A, Mizuguchi M, Kidokoro H, Kurahashi H (2009) Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain and Dev 31:221–227. https://doi.org/10.1016/j.braindev.2008.03.00
Lee JH, Lee M, Lee J (2012) Recurrent acute necrotizing encephalopathy in a Korean child: the first non-Caucasian case. J Child Neurol 27:1343–1347. https://doi.org/10.1177/0883073811435240
Sell K, Storch K, Hahn G, Kirsch L, Ae M, Georgia R et al (2016) Variable clinical course in acute necrotizing encephalopathy and identification of a novel RANBP2 mutation. Brain Develop 38:77–80. https://doi.org/10.1016/j.braindev.2016.02.007
Chew HB, Ngu LH (2020) RANBP2 susceptibility to infection-induced encephalopathy: Clinicoradiologic and molecular description in a Malaysian family. Mol Genetics and Metabolism Reports 24:100627. https://doi.org/10.1016/j.ymgmr.2020.100627
Albayram S, Bilgi Z, Selcuk H, Selcuk D, Cam H, Koçer N, Islak C (2004) Diffusion-weighted MR imaging findings of acute necrotizing encephalopathy. AJNR Am J Neuroradiol 25:792–797
Singh RR, Sedani S, Lim M, Wassmer E, Absoud M (2015) RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur J Paediatr Neurol 19:106–113. https://doi.org/10.1016/j.ejpn.2014.11.010
Howard A, Uyeki TM, Fergie J (2018) Influenza-associated acute necrotizing encephalopathy in siblings. J Pediatr Infect Dis Soc 7:172–177. https://doi.org/10.1093/jpids/piy033
Ohashi E, Hayakawa I, Murofushi Y (2021) Recurrent acute necrotizing encephalopathy in a boy with RANBP2 mutation and thermolabile CPT2 variant: The first case of ANE1 in Japan. Brain Develop 43:873–878. https://doi.org/10.1016/j.braindev.2021.04.009
Shibata A, Kasai M, Hoshinoa A, Tanaka T, Mizuguchi M (2021) RANBP2 mutation causing autosomal dominant acute necrotizing encephalopathy attenuates its interaction with COX1. Neurosci Lett 763:136173. https://doi.org/10.1016/j.neulet.2021.136173
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose. There is no funding support available for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarigecili, E., Ucar, H.K., Havali, C. et al. Acute necrotizing encephalopathy associated with RANBP2 mutation: value of MRI findings for diagnosis and intervention. Acta Neurol Belg 123, 571–582 (2023). https://doi.org/10.1007/s13760-022-02166-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-02166-x