Abstract
Background
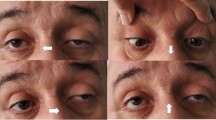
Ocular myasthenia gravis (OMG) constitutes 15% of all myasthenia gravis patients.
Methods
One hundred eight patients with OMG followed-up for over 36 months were retrospectively evaluated regarding factors associated with remission. Demographic features, neuro-ophthalmologic findings at onset, acetylcholine receptor (AChR Ab) and muscle-specifc tyrosine kinase antibodies (MuSK Ab), thymic status, single fiber electromyography (SFEMG) results were the variables considered.
Results
Median age of disease onset was 57 years (range 18–82 years). Clinical features at onset was isolated ptosis in 55 (50.9%) and isolated diplopia in 33 (30.6%) patients. Combined ptosis and diplopia were present in 20 (18.5%) patients. Among 75 patients with ptosis, it was unilateral in 65 (86.7%) and bilateral in 10 (13.3%). AChR Abs were found in 66 (61.1%) and MuSK Abs in 2 (1.9%) patients. SFEMG abnormality was detected in 74 (68.5%) patients. Thymoma was present in 16 (14.8%) and thymic hyperplasia in 6 (5.6%) patients. Forty-one patients (37.9%) had been treated with pyridostigmine alone. Sixty-seven (62%) patients were given immunosupressive drugs. In 53 (49.1%) prednisone was used and in 14 (12.9%) patients it was combined with azathioprine. Thymectomy was performed in all 16 patients with thymoma. Complete stable remission (CSR) was achieved in 49 (45.4%) patients. Fifty-nine (54.6%) patients had reached minimal manifestation (MM) status; 32 (29.6%) having a status of MM-1 and 27 (25%) a status of MM-3.
Conclusions
The presence of AchR Abs (p = 0.034) and an abnormal SFEMG (p = 0.006) at onset as increased risk factors for the presence of ongoing signs necessitating medical treatment.
Similar content being viewed by others
References
Bever CT Jr, Aquino AV, Penn AS, Lovelace RE, Rowland LP (1983) Prognosis of ocular myasthenia. Ann Neurol 14:516–519. https://doi.org/10.1002/ana.410140504
Kaminski H, Maas E, Spiegel P, Ruff R (1990) Why are eye muscles frequently involved in myasthenia gravis? Neurology 40:1663–1669. https://doi.org/10.1212/wnl.40.11.1663
Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14(10):1023–1036
Oosterhuis HJ (1989) The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry 52:1121–1127. https://doi.org/10.1136/jnnp.52.10.1121
Kupersmith MJ, Latkany R, Homel P (2003) Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol 60:243–248. https://doi.org/10.1001/archneur.60.2.243
Grob D, Brunner N, Namba T, Pagala M (2008) Lifetime course of myasthenia gravis. Muscle Nerve 37(2):141–149. https://doi.org/10.1002/mus.20950
Nagia L, Lemos J, Abusamra K, Cornblath WT (2015) Prognosis of ocular myasthenia gravis retrospective multicenter analysis. Ophthalmology 122:1517–1521. https://doi.org/10.1016/j.ophtha.2015.03.010
Hendricks TM, Bhatti MT, Hodge DO, Chen JJ (2019) Incidence, epidemiology, and transformation of ocular myasthenia gravis: a population-based study. Am J Ophthalmol 205:99–105. https://doi.org/10.1016/j.ajo.2019.04.017
Lindstrom JM, Seybold ME, Lennon VA, Whittingam S, Duane DD (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26:1054–1059. https://doi.org/10.1212/wnl.26.11.1054
Evoli A, Alboini PE, Damato V, Iorio R, Provenzano C, Bartoccioni E, Marino M (2018) Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci 1412:82–89. https://doi.org/10.1111/nyas.13518
Peeler CE, De Lott LB, Nagia L, Lemos J, Eggenberger ER, Cornblath WT (2015) Clinical utility of acetylcholine receptor antibody testing in ocular myasthenia gravis. JAMA Neurol 72:1170–1174. https://doi.org/10.1001/jamaneurol.2015.1444
Galassi G, Mazzoli M, Ariatti A, Kaleci S, Valzania F, Nichelli PF (2018) Antibody profile may predict outcome in ocular myasthenia gravis. Acta Neurol Belg 118:435–443. https://doi.org/10.1007/s13760-018-0943-7
Mazzoli M, Ariatti A, Valzania F, Kaleci S, Tondelli M, Nichelli PF, Galassi G (2018) Factors affecting outcome in ocular myasthenia gravis. Int J Neurosci 128:15–24. https://doi.org/10.1080/00207454.2017.1344237
Teo KY, Tow SL, Haaland B, Gosavi TD, Jing-Liang L, Long LOY, Milea D (2018) Low conversion rate of ocular to generalized myasthenia gravis in Singapore. Muscle Nerve 57:756–760. https://doi.org/10.1002/mus.25983
Kamarajah SK, Sadalage G, Palmer J, Carley H, Maddison P, Sivaguru A (2018) Ocular presentation of myasthenia gravis: a natural history cohort. Muscle Nerve 57:622–627. https://doi.org/10.1002/mus.25971
Aguirre F, Villa AM (2018) Prognosis of ocular myasthenia gravis in an Argentinian population. Eur Neurol 79(3–4):113–117. https://doi.org/10.1159/000487132
Katzberg HD, Bril V (2005) A comparison of electrodiagnostic tests in ocular myasthenia gravis. J Clin Neuromuscul Dis 6(3):109–113. https://doi.org/10.1097/01.cnd.0000155026.66153
Fortina E, Cestaria DM, Weinberg DH (2018) Ocular myasthenia gravis: an update on diagnosis and treatment. Curr Opin Ophthalmol 29:477–484. https://doi.org/10.1097/ICU.0000000000000526
Europa TA, Nel M, Heckmann JM (2018) Myasthenic ophthalmoparesis: time to resolution after initiating immune therapies. Muscle Nerve 58:542–549. https://doi.org/10.1002/mus.26172
Benatar M, Mcdermott MP, Sanders DB et al (2016) Eficacy of prednisone for the treatment of ocular myasthenia (EPIT-OME): a randomized, controlled trial. Muscle Nerve 53(3):363–369. https://doi.org/10.1002/mus.2476927
Lee YG, Kim US (2018) Efficacy and safety of low-to-moderate dose oral corticosteroid treatment in ocular myasthenia gravis. J Pediatr Ophthalmol Strabismus 55:339–342. https://doi.org/10.3928/01913913-20180620-01
Kupersmith MJ, Ying G (2005) Ocular motor dysfunction and ptosis in ocular myasthenia gravis: effects of treatment. Br J Ophthalmol 89:1330–1334. https://doi.org/10.1136/bjo.2004.063404
Melson AT, McClelland CM, Lee MS (2020) Ocular myasthenia gravis: updates on an elusive target. Curr Opin Neurol 33:55–61. https://doi.org/10.1097/WCO.0000000000000775
Allen JA, Scala S, Jones H (2010) Ocular myasthenia gravis in a senior population: diagnosis, therapy, and prognosis. Muscle Nerve 41(3):379–384. https://doi.org/10.1002/mus.21555
Wang L, Zhang Y, He M (2017) Clinical predictors for the prognosis of myasthenia gravis. BMC Neurol 17(1):77. https://doi.org/10.1186/s12883-017-0857-7
Li F, Hotter B, Swierzy M, Ismail M, Meisel A, Rückert JC (2018) Generalization after ocular onset in myasthenia gravis: a case series in Germany. J Neurol 265:2773–2782. https://doi.org/10.1007/s00415-018-9056-8
Kısabay A, Özdemir HN, Gökçay F, Celebisoy N (2022) Risk for generalization in ocular onset myasthenia gravis: experience from a neuro-ophthalmology clinic. Acta Neurol Belgica 122(2):337–344. https://doi.org/10.1007/s13760-020-01582-1
Kerty E, Elsais A, Argov Z, Evoli A, Gilhus NE (2014) EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol 21:687–693. https://doi.org/10.1111/ene.12359
Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, Kuntz N, Massey JM, Melms A, Murai H, Nicolle M, Palace J, Richman DP, Verschuuren J, Narayanaswami P (2016) International consensus guidance for management of myasthenia gravis: executive summary. Neurology 87:419–425. https://doi.org/10.1212/WNL.0000000000002790
Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS et al (2000) Myasthenia gravis: recommendations for clinical research standards. Neurology 55(1):16–23. https://doi.org/10.1212/wnl.55.1.16
Gilhus NE (2016) Myasthenia gravis. N Engl J Med 375:2570–2581. https://doi.org/10.1056/NEJMra1602678
Melzer N, Ruck T, Fuhr P et al (2016) Clinical features, pathogenesis, and treatment of myasthenia gravis. A supplement to the guidelines of the German Neurological Society. J Neurol 263:1473–1494. https://doi.org/10.1007/s00415-016-8045-z
Akan O, BaysalKırac L (2021) Ophthalmologic manifestations in myasthenia gravis: presentation and prognosis. Acta Neurol Belg 121:1131–1140. https://doi.org/10.1007/s13760-020-01556-3
Pauda L, Stalberg E, LoMonaco M, Evoli A, Batocchi A, Tonali P (2000) SFEMG in ocular myasthenia gravis diagnosis. Clin Neurophysiol 111:1203–1207. https://doi.org/10.1016/s1388-2457(00)00307-2
Rakocevic G, Moster M, Floeter MK (2017) Single-fiber electromyography in the orbicularis oculi muscle in patients with ocular myasthenia gravis symptoms: does abnormal jitter predict response to treatment? BMC Neurol 17(1):108. https://doi.org/10.1186/s12883-017-0891-5
Fortin E, Cestari DM, Weinberg DH (2018) Ocular myasthenia gravis: an update on diagnosis and treatment. Curr Opin Ophthalmol 29:477–484. https://doi.org/10.1097/ICU.0000000000000526
Benatar M, Kaminski HJ (2007) Evidence report: the medical treatment of ocular myasthenia (an evidence-based review) report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 68:2144–2149. https://doi.org/10.1212/01.wnl.0000263481.14289.90
Evoli A, Bartoccioni E, Lo Monaco M (1988) Ocular myasthenia: diagnostic and therapeutic problems. Acta Neurol Scand 77(1):31–35. https://doi.org/10.1111/j.1600-0404.1988.tb06970.x
Al-Haidar M, Benatar M, Kaminski HJ (2018) Ocular myasthenia. Neurol Clin 36:241–251. https://doi.org/10.1016/j.ncl.2018.01.003
Verma R, Wolfe GI, Kupersmith MJ (2021) Ocular myasthenia gravis—how effective is low dose prednisone long term? J Neurol Sci 420:117274. https://doi.org/10.1016/j.jns.2020.117274
Funding
None.
Author information
Authors and Affiliations
Contributions
Study design and concept: NÇ, FG. Acquisition of the data: AO, FB, HNÖ. Data analysis and interpretation: AO, FB, AKA, NÇ. Manuscript preparation and revision: NÇ, AKA, FG.
Corresponding author
Ethics declarations
Conflict of ınterest
The authors have no conflicts of interest to declare.
Ethics approval and consent to participate
The study protocol was approved by Ege University Medical School Ethics Committee (reference number: 99166796–050.06.04) and was performed in accordance with the ethical standards outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
A written informed consent was obtained from each patient for the publication of this report.
Competing interests
The authors declare that they have no competing interests.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çelebisoy, N., Orujov, A., Balayeva, F. et al. Prognostic predictors of remission in ocular myasthenia gravis. Acta Neurol Belg 123, 1927–1932 (2023). https://doi.org/10.1007/s13760-022-02151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-02151-4