Abstract
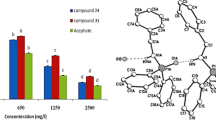
A series of temephos (Tem) derivatives with the general structure of P(O)NH–X–NHP(O) (1–22) were synthesized and characterized by 31P, 13C, 1H NMR and FT-IR spectral techniques. The electron density (ρ) value at the bond critical point (bcp) of P(1′)–O(1′)···(1)H–N(1) (0.040 e Å−3) P(1)–O(1)···(1′)H–N(1′) (0.031 e Å−3) as well as the stabilization energy of electronic delocalization of results of NBO analysis showed the hydrogen bonding energy in P(1′)–O(1′)···(1)H–N(1) model (E 2 = −72.15 kJ mol−1) and P(1)–O(1)···(1′)H–N(1′) (E 2 = −45.67 kJ mol−1) of the crystal cluster 7. The activities of Tem derivatives were evaluated using the modified Ellman’s method on cholinesterase (ChE) enzymes. The insecticide activity of Tem analogous appraised for the elm leaf beetle in which the 18 had more effective than the other compounds in inhibition α-esterase of insect. Principal component analysis–quantitative structure activity relationship (PCA–QSAR) models indicated that it was deduced that the frontier molecular orbital energy parameters in PC1 are predominated from those related to electronic in PC2 and structural parameters in PC3 equation. Multiple linear regressions–QSAR models clarified that the molecular descriptors like an integrated net charge of nitrogen atom (Q N), polarizability (PLN–H) and lowest unoccupied molecular orbital (E LUMO) proved important in defining the activity of the candidates.









Similar content being viewed by others
References
T.K. Olszewski, J. Gałęzowska, B. Boduszek, H. Kozłowski, Eur. J. Org. Chem. 21, 3539 (2007)
V.M.R. Dos Santos, C.M.R. Sant Anna, G.E.M. Borja, A. Chaaban, W.S. Cortes, J.B.N. Da Costa, Bioorg. Chem. 35, 68 (2007)
B. Kaboudin, S. Emadi, A. Hadizadeh, Bioorg. Chem. 37, 101 (2009)
C. Fest, K.J. Schmidt, The Chemistry of Organophosphorus Pesticides (Springer, Berlin, 1982)
E.M. Lores, J.C. Moore, P. Moody, J. Clark, J. Forester, J. Knight, Bull. Environ. Contam. Toxicol. 35, 308 (1985)
S.N. Tikar, A. Kumar, G.B.K.S. Prasad, S. Prakash, Parasitol. Res. 105, 57 (2009)
B.K. Hackenberger, D.J. Perkusic, S. Stepic, Ecotoxicol. Environ. Saf. 71, 583 (2008)
T.A. Ba-Omar, S. Al-Jardani, R. Victor, Tissue Cell 43, 29 (2011)
T. Meiners, M. Hilker, Oecologia 112, 87 (1997)
G.L. Ellman, C.K.D. Outney, V.R. Andres, M. Featherstone, Biochem. Pharmacol. 7, 91 (1961)
PASS software, version 1.917, July (2005)
SPSS for Windows, Version 10.05. SPSS Inc., Bangalore (1999)
G.M. Sheldrick, Acta Crystallogr. A64, 112 (2008)
K. Gholivand, H.R. Mahzouni, Acta Crystallogr. B67, 238 (2011)
A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899 (1988)
J.E. Carpenter, F. Weinhold, J. Mol. Struct. THEOCHEM. 169, 41 (1988)
S.F. Boys, F. Bernardi, Mol. Phys. 19, 553 (1970)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery Jr., T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03, Revision D.01 (Gaussian Inc, Wallingford, 2005)
C. Hansch, T. Fujita, J. Am. Chem. Soc. 86, 1616 (1964)
E.B. de Melo, Eur. J. Med. Chem. 45, 5817 (2010)
P.V. Hentenryck, SAS ‘97, Paris, France. Lecture Notes in Computer Science (1997)
J. Bernstein, R.E. Davis, L. Shimoni, N.L. Chang, Angew. Chem. Int. Ed. 34, 1555 (1995)
K. Gholivand, A.A. Ebrahimi Valmoozi, H.R. Mahzouni, Acta Crystallogr. B69, 55 (2013)
A. Lagunin, A. Stepanchikova, D. Filimonov, V. Poroikov, Bioinform. Appl. Note 16, 747 (2000)
R.A. Copeland, Enzymes, a Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd edn. (John Wiley-VCH, New York, 2000)
S. Soltani, H. Abolhasani, A. Zarghi, A. Jouyban, Eur. J. Med. Chem. 45, 2753 (2010)
J. Singh, B. Shaik, S. Singh, V.K. Agrawal, P.V. Khadikar, O. Deeb, C.T. Supuran, Chem. Biol. Drug Des. 71, 244 (2008)
Acknowledgments
The financial support of Tarbiat Modares, Guilan and Imam Hossein University’s Research Council is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Supplementary data
Supplementary data
CCDC 1027644 and 1027645 contain the supplementary crystallographic data for compounds 1 and 7. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Gholivand, K., Ebrahimi Valmoozi, A.A., Salahi, M. et al. Bisphosphoramidate derivatives: synthesis, crystal structure, anti-cholinesterase activity, insecticide potency and QSAR analysis. J IRAN CHEM SOC 14, 427–442 (2017). https://doi.org/10.1007/s13738-016-0991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0991-y