Abstract
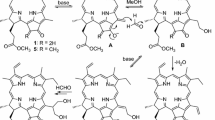
The methyl pheophorbide-a and methyl pheophorbide-b were used as starting materials and converted to purpurin-18 ester by ring-opening and rearrangement reaction in their exocyclic ring. N-Substituted purpurin-18 imides were obtained from purpurin-18 ester through amidation reaction of six-membered cyclic anhydride. Further chemical modifications along their peripheries were carried out by a variety of common reactions, including electrophilic substitution, Wittig reaction, allomerization and Vilsmeier reaction, to afford the title compounds with long-wavelength absorption. The structures of all new chlorins were characterized by elemental analysis, IR, UV–vis and 1H NMR spectra.





Similar content being viewed by others
References
J.J. Wang, Chin. J. Org. Chem. 25, 1353 (2005)
V.Y. Pavlov, G.V. Ponomarev, Chem. Heterocyclic Compt. 40(4), 393 (2004)
A.N. Kozyrev, Y.H. Chen, L.N. Goswami, W.A. Tabaczynski, R.K. Pandey, J. Org. Chem. 71, 1949 (2006)
A.F. Mironov, M.A. Grin, D.V. Dzardanov, K.V. Golovina, Y.K. Shim, Mendeleev. Commun. 11, 205 (2001)
A.P. Castano, T.N. Demidova, M.R. Hamblin, Photodiagn. Photodyn. Ther. 1, 279 (2004)
A.P. Castano, T.N. Demidova, M.R. Hamblin, Photodiagn. Photodyn. Ther. 2, 91 (2005)
G. Zheng, W.R. Potter, S.H. Camacho, J.R. Missert, G.S. Wang, D.A. Bellnier, B.W. Henderson, M.A.J. Rodgers, T.J. Dougherty, R.K. Pandey, J. Med. Chem. 44, 1549 (2001)
Y.H. Chen, G.L. Li, R.K. Pankey, Curr. Org. Chem. 8, 1105 (2004)
A.N. Kozyrev, J.L. Alderfer, T. Srikrishnan, R.K. Pandey, J. Chem. Soc. Perkin Trans. 1, 837 (1998)
A.N. Kozyrev, V. Suresh, M.O. Senge, M. Shibata, T.J. Doughertya, R.K. Pandeya, Tetrahedron. 56, 3353 (2000)
A. N. Kozyrev, J. L. Alderfer, T. J. Doughertya, R. K. Pandey, Chem. Commun. 4, 1083 (1998)
J. J. Wang, G. F. Han, Y. K. Shim, J. Iran. Chem. Soc. 8, 965 (2011)
H. Tamiaki, T. Miyatake, R. Tanikaga, Tetrahedron Lett. 38, 267 (1997)
G.F. Han, J.J. Wang, X.J. Chang, Chin. J. Org. Chem. 24, 197 (2004)
K.M. Smith, D.A. Goff, D.G. Simposon, J. Am. Chem. Soc. 107, 4946 (1985)
J.Z. Li, W.H. Liu, F.G. Li, J.J. Wang, Y.R. Sou, Y.J. Liu, Chin. J. Org. Chem. 27, 1594 (2007)
J. J. Wang, J. Z. Li, F. G. Li J, Iran. Chem. Soc. 8, 1139 (2011)
J.J. Wang, Y.K. Shim, G.J. Jiang, K. Imafuku, J. Heterocycl. Chem. 41, 29 (2004)
J.J. Wang, Y.K. Shim, J.G. Jiang, K. Imafuku, J. Heterocycl. Chem. 40, 1075 (2003)
J. J Wang, P. Wang, J. Z. Li, J. Jakus, Y. K. Shim, Bull. Korean Chem. Soc. 32, 3473 (2011)
J. Wu, J.G. Yin, Q. Zhang, C.M. Sun, F.G. Li, W. Pei, J.J. Wang, Chin. J. Org. Chem. 31, 2011 (2011)
S.H. Lee, N. Jauervic, K.M. Smith, J. Chem. Soc. Perkin Trans. 1, 837 (1993)
G.F. Han, J.J. Wang, Y. Qu, Y.K. Shim, Chin. J. Org. Chem. 26, 43 (2006)
G. Zheng, A. Graham, M. Hibata, J.R. Missert, A.R. Oseroff, T.J. Dougherty, R.K. Pandey, J. Org. Chem. 66, 8709 (2001)
G. Li, A. Slansky, M.P. Dobhal, L.N. Goswami, A. Graham, Y.H. Chen, P. Kanter, R.A. Alberico, J. Spernyak, J. Morgan, R. Mazurchuk, A. Oseroff, Z. Grossman, R.K. Pandey, Bioconjugate Chem. 16, 32 (2005)
X.R. Wu, C. Liu, Z. Yang, N.N. Yao, J.J. Wang, Chin. J. Org. Chem. 32, 632 (2012)
J.G. Yin, Z. Wang, Z. Yang, C. Liu, L.L. Zhao, J.J. Wang, Chin. J. Org. Chem. 32, 360 (2012)
R.G.W. Jinadasa, X.K. Hu, M.G. Vicente, K.M. Smith, J. Med. Chem. 54, 7464 (2011)
J.Z. Li, J.J. Wang, I. Yoon, B.C. Cui, Y.K. Shim, Bioorg. Med. Chem. Lett. 22, 1846 (2012)
J.J. Wang, J.Z. Li, J. Jakus, Y.K. Shim, J. Porphyrins Phthalocyanines 16, 123 (2012)
J.J. Wang, C.L. Liu, J.Z. Li, Synth. Commun. 42, 487 (2012)
J.J. Wang, C.L. Liu, J.Z. Li, Synth. Commun. 43, 487 (2012)
H. Tamiaki, Y. Kotegawa, K. Mizutani, Bioorg. Med. Chem. Lett. 18, 6037 (2008)
R.J. Abraham, K.M. Shimth, D.A. Goff, J.J. Lai, J. Am. Chem. Soc. 104, 4332 (1982)
Acknowledgments
This work was supported by the National Natural Science Foundations of China (No. 21272048) and the Natural Science Foundation of Shandong Province of China (No. Y2008B49).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J.J., Yin, Y.F. & Yang, Z. Synthesis of purpurin-18 imide derivatives from chlorophyll-a and -b by modifications and functionalizations along their peripheries. J IRAN CHEM SOC 10, 583–591 (2013). https://doi.org/10.1007/s13738-012-0194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0194-0