Abstract
Purpose of Review
To examine the epidemiological data on obesity and leukemia; evaluate the effect of obesity on leukemia outcomes in childhood acute lymphoblastic leukemia (ALL) survivors; assess the potential mechanisms through which obesity may increase the risk of leukemia; and provide the effects of obesity management on leukemia. Preventive (diet, physical exercise, obesity pharmacotherapy, bariatric surgery) measures, repurposing drugs, candidate therapeutic agents targeting oncogenic pathways of obesity and insulin resistance in leukemia as well as challenges of the COVID-19 pandemic are also discussed.
Recent Findings
Obesity has been implicated in the development of 13 cancers, such as breast, endometrial, colon, renal, esophageal cancers, and multiple myeloma. Leukemia is estimated to account for approximately 2.5% and 3.1% of all new cancer incidence and mortality, respectively, while it represents the most frequent cancer in children younger than 5 years. Current evidence indicates that obesity may have an impact on the risk of leukemia. Increased birthweight may be associated with the development of childhood leukemia. Obesity is also associated with worse outcomes and increased mortality in leukemic patients. However, there are several limitations and challenges in meta-analyses and epidemiological studies. In addition, weight gain may occur in a substantial number of childhood ALL survivors while the majority of studies have documented an increased risk of relapse and mortality among patients with childhood ALL and obesity. The main pathophysiological pathways linking obesity to leukemia include bone marrow adipose tissue; hormones such as insulin and the insulin-like growth factor system as well as sex hormones; pro-inflammatory cytokines, such as IL-6 and TNF-α; adipocytokines, such as adiponectin, leptin, resistin, and visfatin; dyslipidemia and lipid signaling; chronic low-grade inflammation and oxidative stress; and other emerging mechanisms.
Summary
Obesity represents a risk factor for leukemia, being among the only known risk factors that could be prevented or modified through weight loss, healthy diet, and physical exercise. Pharmacological interventions, repurposing drugs used for cardiometabolic comorbidities, and bariatric surgery may be recommended for leukemia and obesity-related cancer prevention.
Similar content being viewed by others
Introduction
Leukemia constitutes a collection of blood-related malignancies characterized by the transformation of hemopoietic progenitors and the diffuse infiltration of the bone marrow. According to the Fourth Edition of the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues, leukemia can be broadly categorized into myeloid or lymphoid lineages [1]. Based on the course of disease progression (acute or chronic) and the origin of the predominant cell type (lymphoid or myeloid), leukemia is classified into four main types: acute lymphocytic leukemia (ALL), acute myelogenous leukemia (AML), chronic lymphocytic leukemia (CLL), and chronic myelogenous leukemia (CML). Worldwide, based on the GLOBOCAN database, leukemia is estimated to account for approximately 2.5% and 3.1% of all new cancer incidence and mortality in 2020, respectively [2]. Moreover, leukemia represents the most frequent cancer in children younger than 5 years of age accounting for the highest percentage of deaths in this age group [3]. The majority of leukemia cases in childhood are acute, and ALL is the most common type in pediatric populations globally [4].
The etiology of most cases of leukemia has not been elucidated. Leukemia is a multifactorial disease stemming from the interaction of genetic, epigenetic, and environmental factors. Age represents a significant risk factor for cancer including leukemia [5, 6]. With the exception of ALL, leukemia dramatically increases with age, peaking at 80 to 85 years old (y.o.), with a median age at diagnosis between 65 and 72 y.o. [7]. A number of suggested and established risk factors have been implicated, including genetic disorders, certain blood disorders, exposure to ionizing radiation, chemicals such as benzene, and pesticides, infections, cancer treatment with radiotherapy, and/or mutagenic chemotherapy and family history [4, 5, 8,9,10]. Tobacco smoking and alcohol consumption have also been documented as risk factors in several studies, whereas recent studies have indicated that obesity may contribute to the etiopathogenesis of leukemia [5, 11]. Obesity constitutes a disorder of energy homeostasis which manifests as excessive adipose tissue accumulation [12,13,14]. As there are no biological markers of overweight and obesity to date, they are diagnosed based on the body mass index (BMI), which is the best and most practical screening test [15,16,17]. Using the WHO criteria, overweight and obesity are defined as a BMI ≥ 25 and ≥ 30 kg/m2 [18]. However, BMI is not the perfect measure, mainly because it does not provide information on the distribution of the adipose tissue (visceral versus subcutaneous), being also insensitive to the ratio of fat to muscle [19, 20]. The global prevalence of obesity has risen dramatically, with more than 670 million adults being obese. It is estimated that, worldwide, almost 39–49% of the global population (around 2.8 to 3.5 billion individuals) has overweight or obesity [21, 22]. Furthermore, childhood obesity represents a global pandemic [23]. Obesity has been associated with a plethora of disorders, including metabolic syndrome, hypertension, type 2 diabetes, cardiovascular disease and risk factors, non-alcoholic fatty liver disease, sleep disorders, polycystic ovary syndrome as well as the severity of COVID-19 and cancer [24,25,26,27].
Based on the International Agency for Research on Cancer (IARC) Working Group, there is convincing evidence that excess body weight is associated with an elevated risk for malignancies of at least 13 anatomic sites, including endometrial, esophageal, renal, and pancreatic adenocarcinomas; hepatocellular carcinoma; gastric cardia cancer; meningioma; colorectal, postmenopausal breast, ovarian, gallbladder, and thyroid cancers as well as multiple myeloma [28, 29]. Moreover, there is a strong indication that obesity may be associated with the incidence and mortality of leukemia, particularly AML, CLL, CML, and ALL as well as preleukemic conditions such as myelodysplastic syndromes (MDSs) [11, 30,31,32,33,34,35].
Whereas obesity may be associated with leukemia based on epidemiologic studies, the biologic rationale and the mechanisms underlying this link remain largely obscure. The goal of this review is to provide an overview of the association between excess body weight and leukemia summarizing important biological mechanisms underpinning this relationship as well as underscoring recent developments on novel insights in pathogenetic mechanisms. Moreover, we give a special emphasis on current epidemiologic evidence and its limitations; the role of bone marrow adiposity in leukemia pathogenesis; the association between obesity and childhood ALL survivors; as well as preventive and therapeutic perspectives and challenges.
Methodology of the Review
In June 2023, a literature search in the PubMed database was conducted to assess the association between obesity and the risk of leukemia. This search used the following MESH terms: “Obesity” AND “leukemia” AND “risk.” A search of the abovementioned terms yielded a total of 540 results, most of which were published during the past 10 years. Among the 540 studies, 11 were excluded as 3 were written in Polish, 3 in Russian, 2 in Spanish, 2 in Chinese, and 1 in Czech. In addition, 14 studies dealt with cardiovascular (CVD) risk, 14 studies with hyperglycemia and/or insulin resistance, 8 studies referred to nutritional aspects, such as tea or caffeine consumption, 8 studies with other hematologic malignancies (4 with multiple myeloma, 4 with lymphomas), 5 studies with venous thromboembolism events, 5 studies were case reports, and 8 studies dealt with genes and neurological aspects. Therefore, from the 540 studies, 73 studies were excluded, leaving a total of 467 studies.
Epidemiologic Evidence Linking Obesity to Leukemia
Evidence from Epidemiologic Studies and Meta-Analyses
Current evidence has suggested a relationship between obesity and leukemia. Indeed, Bhaskaran et al. have documented a significant association between obesity and the risk of leukemia, in their landmark study including 5,240,000 adults, that was published in the Lancet in 2014. In particular, they reported that a 5 kg/m2 increase in BMI was almost linearly related to an increased risk of leukemia, among other cancer types [36]. Estimates from the Global Burden of Disease Study, which analyzed data from 1990 to 2017 globally, have reported a significant association between higher BMI and an increased risk of AML [37]. In addition, in 2022, Huang et al. have reported a significant association between obesity and the risk of leukemia [38]. Moreover, in 2023, Ahmed et al. have studied the incidence of various types of cancer among 290,888 participants from the UK BioBank. Totally, 21,973 participants aged 37 to 73 years old, with a median follow-up of approximately 4 years, developed cancer. They concluded that a metabolic profile characterized by an increased BMI in conjunction with increased serum C-reactive protein (CRP) and cystatin C levels may predict an elevated risk of hematologic malignancies in middle age and older people [39]. A broad-scale analysis of cancer-related deaths in the USA between 1982 and 1999 revealed that among other malignancies, a dose-response relationship between BMI and leukemia mortality likely exists, with increasing death rates across overweight, class I, II, and III obesity compared to lean individuals, respectively. These observations were independent of important confounders such as age, nutritional factors, physical activity, tobacco, and alcohol consumption, among others; however, no information regarding different leukemia subtypes was provided [40]. Table 1 depicts major studies associating obesity with an increased risk of leukemia. Overall, there are many studies supporting an association between obesity and an increased risk of all types of leukemia (lymphocytic versus myeloid, acute versus chronic). The presence of obesity is associated with an increased risk of essentially the sum of conditions falling into the spectrum of leukemic disease; this includes CLL and CML, ALL and AML [31], as well as the pre-malignant myelodysplastic syndromes [49]. Although evident in all the aforementioned conditions, the added risk conferred by obesity is likely greater for acute leukemias, especially of lymphoid but also of myeloid origin [31, 50] compared with chronic leukemias. The role of obesity as a risk factor for specific subtypes of AML remains to be fully elucidated; a particularly strong association has been observed for acute promyelocytic leukemia (APL), with an additional 44% risk for each 5 kg/m2 increase in BMI [51]. Furthermore, it is unclear whether the putative underlying pathogenetic mechanisms linking obesity to leukemogenesis, which are expanded upon in the following sections, are homogenously implicated in all leukemia subtypes; it is likely that a number of mechanisms are common, whereas others (e.g., perturbations of bone marrow adipose tissue physiology) tend to more selectively partake in the pathogenesis of specific leukemias (in this case, of myeloid origin).
Birth Weight and Childhood Leukemia
There is a growing body of evidence suggesting that an increased birthweight (BW), usually defined as ≥ 4000 g, may be associated with the development of childhood leukemia. This association may be attributed to increased levels of growth hormone (GH) and insulin-like growth factors (IGF) in infants, who have later developed leukemia [52, 53]. As GH and IGF are also related with an increased stature, it has been postulated that increased height at diagnosis of ALL may be observed among children with ALL [54]. However, although Huang et al. have reported this positive association between height at diagnosis and ALL, later studies have questioned this relationship [55]. Schraw et al. have attributed these apparently different findings to the selection of distinct populations studied in diverse clinical settings and with different reference data used [55]. Nevertheless, even though height at diagnosis of childhood ALL may not be a consistent finding, BW seems to be a risk factor for developing childhood ALL [56]. In addition, newborns larger for gestational age were documented to be at a higher risk for childhood ALL [57]. Table 2 depicts main studies associating elevated BW with an increased likelihood of childhood leukemia. Interestingly, a body of epidemiological studies, including a recent meta-analysis, have documented a relationship between maternal obesity and leukemia in the offspring; nevertheless, the pathogenetic mechanisms underlying this relation are unclear [61, 62].
Limitations of Epidemiologic Studies and Meta-Analyses
Notwithstanding that most studies have depicted a relationship between obesity and the risk of leukemia, there are several limitations in meta-analyses and epidemiological studies. First, when interpreting the included meta-analyses, the inherent limitations of the original studies should be taken into account. For example, the main indicator of obesity, i.e., BMI measurement, was inconsistent with variations from WHO-specified criteria while self-reported questionnaires were commonly used instead of objective measures, which may have influenced the accuracy of the results. In addition, BMI is a practical measure of obesity, but has some inherent drawbacks. It has been demonstrated that BMI does not reflect the body fat distribution and the subsequent CVD risks associated with adiposity [19, 20, 57, 63]. Moreover, other studies have shown decreased response rate amid control participants, small number of included studies, and limited statistical power. Many studies were retrospective which are prone to selection bias in comparison to cohort studies. Other investigations have shown heterogeneous results, while publication bias must be taken into account in systematic reviews [64]. Therefore, limitations exist when comparing different studies; nevertheless, the general tendency of an association between obesity and leukemia risk should not be overlooked.
Obesity and Childhood ALL Survivors
Weight Gain Among Survivors of Childhood
Despite the rise in overweight/obesity rates in childhood as well as in adulthood, most children with ALL have normal weight at diagnosis of ALL. However, during or after treatment of childhood ALL, substantial increases in weight have been documented. More specifically, as many as 50% of childhood ALL survivors have increased body weight and this weight gain has been attributed to multiple factors [65].
The Childhood Cancer Survivor Study (CCSS) was conducted by 26 medical centers in the USA and Canada enrolling more than 14,000 cancer survivors, who were diagnosed between 1970 and 1986 [66]. The CCSS has reported a 20% increase in obesity among males and a 50% increase among female survivors [66]. In addition, in a meta-analysis among 9223 pediatric ALL survivors, Zhang et al. have concluded that obesity was much more prevalent in ALL survivors, when compared to the reference group [67]. More specifically, the majority of studies had enrolled survivors who were off treatment for less than 5 years, whereas only a small number of studies included survivors who were off treatment for more than 10 years. In particular, among patients who were off treatment for at least 10 years, prevalence of obesity was between 34% and 64% [67]. It is noteworthy that subgroup analysis has demonstrated obesity to be more prevalent regardless of their age at onset of ALL, their gender, or the previously administered cranial radiation therapy (CRT) or not [67]. Very recently, Richard et al. have reported the results from the CCSS and the St. Jude Lifetime Cohort (SJLIFE) studies regarding genetic variants in adult survivors of childhood ALL [68•]. By using Genome-Wide Association Study (GWAS), they have documented that more than 700 loci are responsible for 6.2% of the genetic variation of BMI in adult survivors of childhood ALL. They have confirmed that ALL survivors have approximately the same genetic heritability as the general population regarding BMI. However, CRT may modify BMI-associated loci among adult survivors of childhood ALL [68•]. Furthermore, Green et al. have shown that CRT, physical inactivity, and the use of certain anti-depressant medication are correlated with increased BMI among pediatric ALL survivors in adulthood [69]. In an Epigenome-Wide Association Study (EWAS), Wahl et al. have documented that variations in BMI, as a marker of adiposity, are correlated with changes in DNA methylation at cytosine-guanine sites [70]. Indeed, Lupo et al. have studied 96 pediatric ALL survivors and have shown that 39 loci were related to obesity among the pediatric ALL survivors, who received only chemotherapy and not CRT [71••]. Therefore, with the use of WGAS and EWAS, researchers are now able to confirm that different molecular pathways are involved in the development of obesity among pediatric ALL adult survivors, who received only chemotherapy or only CRT.
Overall, weight gain may occur in a substantial number of childhood ALL survivors via different molecular pathways. Chemotherapy, CRT, and the administration of corticosteroids may be implicated in the development of obesity among pediatric ALL survivors.
Relationship Between Excess Body Weight and Risk of Relapse and Mortality
There is a growing body of evidence supporting the notion that adiposity is associated with a decreased efficacy of ALL treatment [72]. This notion is based upon the fact that lymphoblasts have been documented to migrate into the adipose tissue [73]. Notably, lymphoblasts in the adipose tissue are protected from degradation, while adipose-derived stromal/stem cells secrete factors, which have been implicated in the proliferation of lymphoblasts [74]. Thus, the combination of proliferation and protection of lymphoblasts in the excess adipose tissue accounts for the increased risk of mortality in ALL patients, which has been attributed to obesity [74, 75]. More specifically, adipocytes secrete lipids and amino acids, which support the growth and proliferation of leukemia initiating cells (LICs) [76, 77]. In addition, Lee et al. have reported that adipocytes may induce the expression of Galectin-9 (GAL-9) on the surface of B-ALL cells in humans [78]. They have confirmed the enhanced expression of GAL-9 on B-ALL cells among pediatric patients with obesity, when compared to lean patients with pediatric B-ALL [78]. They have also documented that in relapse, higher gene expression of GAL-9 has been correlated to poorer outcomes [78]. Therefore, increased GAL-9 expression may exert “protective effects” on B-ALL cells [78]. Moreover, apart from the protective effects of adipocytes on LICs, obesity could induce alterations in the pharmacokinetics of various chemotherapeutic agents through multiple mechanisms. For example, the accumulation of lipid-soluble chemotherapeutics and the enhancement in the secretion of water-soluble drugs may lead to changes in the metabolism of chemotherapeutic agents [79]. Besides, further metabolism of chemotherapeutic agents, such as doxorubicin and daunorubicin via reductases, may also contribute to changes in the efficacy of the abovementioned anthracyclines [80].
Higher adiposity, chiefly indexed by increased BMI, has been associated with adverse leukemia outcomes in adults, although the overall findings are controversial, and the effects are likely lineage specific. In a recent study among adolescent and adult younger ALL patients aged younger than 50 years, elevated BMI was independently associated with increased treatment toxicity (mainly hepatotoxicity and hyperglycemia), higher relapse-free mortality, and shorter overall survival, effects which were more pronounced among the higher age groups [81•]. An earlier report demonstrated an increased 5-year mortality among adults with ALL and obesity (HR 1.60, 95% CI 1.03–2.50, p = 0.035). This was exclusively driven by the increased mortality in the subset of patients with ALL of T-lymphocyte lineage (HR 5.42, 95% CI 1.84–15.98, p < 0.001), while no impact of BMI was observed on patients with B-ALL [82]. On the other hand, among 1974 newly diagnosed cases of AML in adult patients, obesity was associated with better rates of complete remission and lower incidence of treatment-resistance AML, without any noted effects on survival [83]. In contrast, another study reported worse overall survival among patients with AML and obesity (aHR 0.6, p = 0.03), a finding which was independent of comorbidity burden, age, cytogenetic features of AML, or treatment intensity [84]. The discrepant findings regarding the impact of obesity on AML prognosis may at least partially be attributable to the differential effects according to AML subtypes. Based on a relevant meta-analysis, obesity seems to adversely affect prognosis particularly in APL [85].
Table 3 depicts major studies examining the association between obesity and the risk of relapse or mortality among patients with pediatric ALL. The majority of studies have documented an increased risk of relapse as well as increased mortality rates among patients with childhood ALL and obesity [75, 89, 91]. However, a minority of studies have not confirmed this correlation [86]. The discrepancy of results may be due to differences in ethnic groups. Of note, Mexican children with ALL possess ETV6-RUNX1 in only about 6%, a gene rearrangement, which seems to be related to a better outcome, whereas in developed countries, this gene rearrangement is present in 22% approximately [92]. Apart from differences in ethnic groups, different sample sizes as well as variations in confounding factors, adjustments in statistical analyses and median follow-up times may all be associated with discrepancies of results. Therefore, further large-scale studies are needed to confirm the relationship between increased risk of relapse and mortality among obese patients with childhood ALL, when compared to normal weight patients with ALL.
Biological Mechanisms Associating Obesity with Leukemia
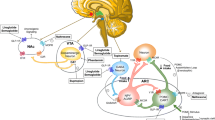
Aside from the main properties of the adipose tissue which encompass energy storage and thermal insulation, the adipose tissue is the largest endocrine organ that secretes a plethora of bioactive polypeptides, called “adipokines” or “adipocytokines” [16]. White, beige/brite, brown, and pink fat tissues represent the main types of adipose tissue, while all four types of adipocytes have endocrine functions [93,94,95]. Adipocytes are also present in the bone marrow and marrow adipose tissue (BMAT) representing about 10% of the human organism’s total fat tissue mass [96]. Obesity may lead to an enlargement of the BMAT size [97]. Interestingly, in the bone marrow milieu, there exists a network among leukemic blasts, hematopoietic stem cells (HSCs), adipocytes, pre-adipocytes, and other cells, such as osteoblasts, osteoclasts, and osteocytes via signaling molecules [98, 99]. The pathophysiological mechanisms linking obesity to leukemia are presented in Fig. 1. Although the role of excess body weight in leukemia etiopathogenesis is not fully elucidated, and the main pathways linking obesity adiposopathy to leukemia are complicated and comprise BMAT; hormones including insulin and the insulin-like growth factor system as well as sex hormones; pro-inflammatory cytokines and growth factors, such as IL-6 and TNF-α; adipocytokines, such as adiponectin, leptin, resistin, and visfatin; dyslipidemia and lipid signaling; chronic low-grade inflammation and oxidative stress; and other emerging mechanisms.
The pathophysiological mechanisms linking obesity to leukemia. AAs, aminoacids; BM, bone marrow; ER, estrogen receptor; FFAs, free fatty acids; HDL, high-density lipoprotein; IGF, insulin-like growth factor; Ins, insulin; InsR, insulin receptor; IRS, insulin receptor substrates; LDL, low-density lipoprotein; ROS, reactive oxygen species. All images are originated from the free medical site http://smart.servier.com/ (accessed on August 7, 2023) by Servier licensed under a Creative Commons Attribution 3.0 Unported License
Insulin, Insulin Resistance, and the IGF-1 Axis
Insulin resistance represents a pathological state defined as a condition of lower insulin-targeting tissue responsiveness to insulin levels [100,101,102]. Obesity constitutes a chronic hyperinsulinemic state, and when insulin secretion can no longer compensate for insulin resistance, metabolic syndrome and type 2 diabetes mellitus (DM) may develop [27, 35, 103]. Chronic hyperinsulinemia is related with an elevated risk of several obesity-related cancers, such as breast, endometrial, ovarian, and prostate cancers [104,105,106,107,108].
Multiple levels of the signaling pathways of insulin and IGF-1 are of capital importance in the pathogenesis of leukemia. Under normal conditions, healthy cells, including lymphocytes, exhibit low levels of surface insulin receptor (InsR) expression, due to the degradation of InsR following insulin binding [109]. The upregulation of membrane InsR has been recognized as a tumorigenesis-promoting mechanism in certain solid malignancies and CLL [110], although corresponding evidence regarding acute leukemias is lacking [111]. Likewise, IGF-1 receptor in T-ALL is maintained high by Notch signaling [112], and is induced in B-ALL by HoxA9 overexpression and occasionally in AML, thus promoting leukemogenesis [113, 114].
Following insulin or IGF-1 binding to their receptors, further signal transduction involves the phosphorylation of the insulin receptor substrates (IRS) and the subsequent activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin (PI3K/Akt/mTOR) pathway [115]. Furthermore, cytokine as well as steroid and other hormone receptors and integrins also utilize IRS phosphorylation in order to regulate cellular metabolism, growth, differentiation, or proliferation [116]. This particular role of IRS as effectors of versatile extracellular signals, which include not only insulin and IGF-1 but also interleukins (ILs) and other cytokines elevated in the systemic environment of chronic low-grade inflammation observed in obesity and insulin resistance, renders them and their related pathways key intersection points in the pathogenesis of leukemic disease in obesity. Intracellular signaling involving the IRS1/2 is implicated in normal hematopoiesis, and perturbation of the IRS expression and/or its phosphorylation status have been implicated in leukemogenesis [117]. The IRS1/2 signaling interrelates with the BCR/ABL [118] or JAK2 pathways in chronic myeloproliferative disorders [119]. Activating mutations of the IRS-2 have been implicated in the pathogenesis of chronic myeloid leukemia refractory to tyrosine kinase inhibitor treatment [120]. The knockdown of IRS1/2 or their targeting by overexpression of miR-570 suppresses glucose metabolism, inhibits proliferation, and induces apoptosis of CML cells in vitro [121]. Likewise, the IRS-2 overexpression is observed in patients with non-CML chronic myeloproliferative disorders; the silencing of IRS-2 reduced cell viability and increased apoptosis in cells harboring the pathogenetic JAK2V617F mutation and enhanced the effects of JAK1/2 inhibitor ruxolitinib [122]. Mutations of IRS2 have also been identified in chronic myeloproliferative neoplasms which do not exhibit the most common mutation in JAK2, MPL, and CALR genes [123]. IRS1 is also overexpressed in ALL cells compared with normal hematopoietic cells, despite similar levels of IGF-1R expression [124]. Increased expression of IRS1 in adult BCR/ABL-positive B-ALL is associated with lower survival independently of age and leukocyte count at diagnosis [125]. Similarly, in vitro treatment of pre-B-ALL cells with BCR-ABL inhibitor GZD824 downregulates IRS-1 and the subsequent activation of the PI3K/AKT pathway, inducing cell cycle arrest and promoting apoptosis [126].
The downstream proliferative and anti-apoptotic effects of insulin and IGF-1 signaling are mediated by the activation of the PI3K/Akt/mTOR and RAS/RAF/MAPK/ERK pathways [127, 128]. Subsequently, targeting components of these pathways via newly developed agents or repurposed drugs from the obesity/type 2 diabetes armamentarium constitutes an attractive putative strategy in the treatment of leukemic disease [113, 114, 129,130,131] (see also “Candidate Therapeutic Agents Targeting Oncogenic Pathways of Obesity and Insulin Resistance in Leukemia” section). Moreover, in ALL, hyperglycemic patients undergoing induction have shorter durations of remission and median survival compared to normoglycemic patients [132]. Mechanistic studies have suggested high levels of insulin/insulin signaling as the underpinning mechanism of this finding [133].
Distorted IGF-1/IGF-1R signaling has been linked to the development of aggressive and/or refractory leukemia [114, 134,135,136]. In the context of pediatric AML, dysregulation of this pathway has been associated with treatment failure and decrease relapse-free survival in both the setting of induction chemotherapy and HSC transplantation [134,135,136]. A study in 30 AML patients showed that elevated serum levels of the insulin-like growth factor binding protein (IGFBP) family were associated with worse progression-free survival and overall survival, suggesting that outcomes in myeloid leukemias may be influenced by IGFBPs, probably mediated through the alteration of IGF-1R activation [136]. In a broader context, it has been suggested that AML cells native to the adipose tissue of affected patients may induce the production of IGFBP1 by adipocytes leading to a state of systemic insulin resistance and directly act as a mitogenic signal on CML cells through the Erk signaling pathway [137].
In summary, alterations of InsR and IGF-1r signaling are observed in various leukemia types, which ultimately contribute to the leukemic cell proliferation, evasion of apoptosis, and/or resistance to treatment. Furthermore, components of these pathways may serve as potential targets for anti-leukemic treatment.
Dyslipidemia and Lipid Signaling
Obesity is accompanied by atherogenic dyslipidemia, which is characterized by quantitative and qualitative changes of plasma lipoproteins [138,139,140]. The major dysregulations in the lipid profile comprise hypertriglyceridemia, reduced high-density lipoprotein (HDL) cholesterol level, and elevated small dense low-density lipoprotein (LDL) particles [138]. Dyslipidemia has also been linked to high cancer incidence and mortality in solid tumors [141]. Early findings have suggested that patients with hematologic malignancies display lipid profile abnormalities that are proportional to the tumor burden [142]. The Metabolic Syndrome and Cancer Project that focused on 578,000 adults identified that total cholesterol and triglyceride levels are inversely correlated with the incidence of myeloid neoplasms [143]. Since then, and based on this premise, statin therapy has been used in efforts to increase chemotherapy efficacy in AML with promising results [144, 145]. However, these studies were phase I/II trials, and further investigation in the randomized setting is warranted.
Lipid signaling is one potential pathway through which obesity may promote cancer. Obese people exhibit higher concentrations of circulating free fatty acids (FFAs), prominently as a manifestation of adipocyte insulin resistance which results from the failure of circulating insulin to suppress lipolysis [146], leading further to the aggravation of insulin resistance in peripheral tissues [147]. On the other hand, the presence of obesity and related conditions is associated not only with elevated concentrations but also qualitative shifts in the circulating FFA pool [148, 149], which may differentially affect the FFA receptor activity [150].
Alterations of circulating FFA profiles are also observed in acute leukemias and pre-leukemic conditions such as myelodysplastic syndromes and aplastic anemia [151]. Furthermore, adipocytes exhibit a release of FFAs in the presence of ALL cells, which are in turn stored intracellularly in leukemic cells for on-demand energy production or act as building blocks for the production of other macromolecules [152]. Leukemic cells in relapsed AML exhibit aberrant lipid metabolism, with increases of highly unsaturated and long-chain fatty acids, sphingomyelins, and triglycerides, among others [153].
The monoacylglycerol lipase pathway may promote the upregulation of FFAs in cancer cells [154]. These fatty acids could be turned into tumorigenic signaling lipids, through the fatty acid synthase [155]. These lipid signaling molecules include lysophosphatidic acid, prostaglandins, sphingosine-1-phosphate (S1P), platelet activating factor, and phosphoinositides, which may promote tumorigenic pathways including proliferation, invasiveness, and aberrant immunological response [156]. S1P is of particular interest for leukemia as it stimulates the growth and survival of leukemia and lymphoma cells through the NF-kappa B pathway [157].
Elevated circulating triglyceride and lower HDL-cholesterol levels have been consistently observed in ALL and AML [158,159,160,161,162]. Accordingly, overall survival in AML patients has been shown to be independently associated with elevated triglyceride and reduced HDL levels before treatment initiation [163]. Of note, this pattern is consistent with the so-called diabetic dyslipidemia, which likely emerges as a result of insulin resistance and increased production of large buoyant VLDL1 particles by the liver [164, 165]. Hence, it would seem possible that the observed associations may be mediated by the presence of insulin resistance and not as a direct consequence of altered lipoprotein levels. In this regard, a retrospective study among 712 newly diagnosed AML cases (319 acute promyelocytic and 393 non-promyelocytic) has shown the presence of elevated triglycerides and lower HDL as risk factors for higher initial leukocyte counts and early death in APL. Furthermore, it highlighted the role of the increased peroxisome proliferator-activated receptor alpha (PPARα) expression as a common denominator for increased triglycerides and leukemic cell proliferation [166].
Sex Hormones
Increased adipose tissue affects sex hormone physiology in both genders [167]. With excess body weight, the levels of testosterone diminish in men with obesity, whereas obese women, especially those with an abdominal phenotype, may present a state known as functional hyperandrogenism [168, 169]. Epidemiological studies have found that the incidence of hematological malignancies varies depending on the sex. Since males are about twice as likely to be diagnosed with ALL or CLL and other lymphomas, it has been hypothesized that estrogen may act as a preventative factor in the onset of these neoplasms [170, 171]. Estrogen signaling pathways have recently been implicated in normal hematopoiesis [172].
Estrogen receptor alpha (ERα) induces cells to growth and is expressed throughout the body, including the hematopoietic tissue [173]. On the other hand, estrogen receptor beta (ERβ) exerts anti-proliferative effects and is expressed in the bone marrow, lung, colon, breast, and prostate. In the context of blood cancers, researchers found that the ERα CpG island is abnormally methylated in a big proportion of all malignant neoplasms and ~ 90% of samples of AML patients [174]. This methylation pattern is mostly observed in normal karyotype AML and leads to the downregulation of ERα expression. However, there is conflicting evidence about the role of gene methylation and long-term patient outcomes. About a third of the genes commonly associated with AML biology have been shown to be upregulated by ERα [175]. It is very difficult to establish causality of methylation and carcinogenesis as the genetic alterations in AML often affect the epigenetic landscape of the blasts.
In AML, ERβ is more highly expressed than ERα in some AML patient gene sets [176, 177]. High ERβ/ERα ratios may contribute to the potential role of ERβ signaling against leukemia [178]. Nevertheless, data on the impact of ERβ signaling are limited while its role is not clear.
Chronic Inflammation and Oxidative Stress
Chronic systemic low-grade inflammation is a hallmark feature of obesity and insulin resistance. A multitude of mechanisms contribute to an increase of circulating levels of pro-inflammatory cytokines in obesity, in conjunction with adipose tissue inflammation, dysfunction and hypoxia, and deteriorating insulin resistance [179] (see also “Dysregulation of Cytokines and Adipokines” section). Concurrently, a spectrum of pro-inflammatory cytokine levels overlapping with those elevated in obesity appear increased ALL and AML [180, 181], while aberrant cytokine signaling is a consistent pathogenetic feature of cell proliferation, survival, and resistance to chemotherapy in leukemia [182]. Hence, a plausible hypothesis could implicate the mitogenic activation of hematological progenitors and/or leukemic cells by the chronically elevated cytokines in obesity as a putative link between increased adiposity and leukemogenesis. According to a recent study, central obesity indexed by an elevated waist-to-hip ratio is associated with the presence of clonal hematopoiesis of indeterminate potential [183], a condition associated with a yearly risk of 0.5–1% for leukemia [184]. Mechanistic data from the same study has revealed that this relationship is likely mediated by the excessive inflammatory environment accompanying increased adiposity [183].
In the context of AML, chronic inflammation is a feature of MDS progression to AML [185]. It has been shown that inflammatory cytokines can promote progression to leukemia in vivo [186]. In myeloid malignant cells, innate immune signaling is often erroneously amplified, an effect mediated through the toll-like receptors (TLRs) that physiologically senses pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) and promotes an inflammatory response [187]. The activated TLR axis results in the secretion of several cytokines from leukemic cells that increases cell viability [188]. The mutational landscape of preleukemic states and AML (e.g., DNMT3A, TET2) may also make the HSCs vulnerable to inflammatory signals that promote leukemogenesis [189]. A very recent study in AML patients described unique inflammatory signatures that correlate with worse prognosis [190]. These were derived from single-cell level data and comprised of atypical B cells, a dysfunctional B cell subtype, an increase in CD8+ GZMK+, an elevation of regulatory T cells, and the concurrent decrease in T cell clonal expansion. The authors have also created an “inflammatory gene” score that correlates with survival outcomes in patients with AML.
However, it is important to note that the effects of inflammatory signaling are context dependent [188]. Based on the cellular molecular and chemical context, the activation of one inflammatory pathway may lead to malignant clonal expansion, the activation of an alternative pathway or secretion of a cytokine might lead to clonal suppression, and other pathways might be passengers in the disease course.
Interestingly, the abundance of main energy substrates in the context of obesity, namely glucose and FFA, leads to the overloading of intracellular energy provision pathways, an overproduction of NADH and FADH2 carrying electrons in the mitochondrial respiratory chain, and the production of reactive oxygen species during cellular respiration at rates exceeding the neutralizing capacity of cellular antioxidant mechanisms [102]. This imbalance leading to the accumulation of reactive oxygen species (ROS) is referred to as oxidative stress. Oxidative stress is an inherent feature of obesity and insulin resistance (IR) [191]. Oxidative stress is considered to play a major role in carcinogenesis by inducing base modification and DNA damage leading to mutations of proto-oncogenes and tumor suppressor genes [192]. The precise role of oxidative stress in leukemogenesis remains controversial, while additionally, the application of ROS to induce blast cell death has been considered in the treatment of leukemia [193].
Dysregulation of Cytokines and Adipokines
The adipose tissue exhibits diverse endocrine functions, being a source of numerous hormonally active molecules, collectively referred to as adipokines and, more specifically, adipocytokines to denote pro-inflammatory cytokines originating from the adipose tissue [194, 195]. The secretory and circulating profiles of these molecules are subject to the distribution of adipose tissue of origin (visceral or subcutaneous) and substantial changes from the lean state to increasing obesity severity [196]. HSCs are the epicenter of a careful balance between quiescence, self-renewal, and differentiation within the healthy BM milieu [197]. In inflammatory states, many cytokines, including IL-1, IL-3, IL-6, tumor necrosis factor-a (TNF-a), and interferon (IFN) together with several growth factors such as M-CSF, G-CSF, and GM-CSF, drive the equilibrium from the steady state to emergency hematopoiesis [198]. The dysregulation of cytokine secretion is a hallmark of leukemia and preleukemic states [198,199,200]. Several studies have shown that IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-27, IL-35 as well as GM-CSF and stem cell factor (SCF) are elevated in AML patients compared to healthy controls [186, 201,202,203,204]. IL-1b can stimulate the generation of cytokines such GM-CSF and IL-6, acting as an autocrine growth factor for AML blasts [198, 205]. Besides, pro-inflammatory adipocytokines could directly promote leukemic cell survival and/or resistance to treatment; this may in turn harbor important implications for potential therapeutic approaches. Inhibition of IL-1 signaling constitutes a prominent example. An endogenous IL-1β repressor cytokine and likewise the monoclonal antibody canakinumab reduce leukemic cell proliferation in AML xenografts [206]. AML blasts overexpress IL-1 receptor accessory protein (IL-1RAP), an indispensable component of IL-1 receptor-related signaling. Chimeric antigen receptor T cells or monoclonal antibodies targeting IL-1RAP exhibit cytotoxic activity and inhibit the proliferation of AML cells, respectively [207, 208].
The presence of obesity also exerts a significant impact on the circulating profile of main adipokines. Increases of leptin, resistin, and visfatin as well as decreases of adiponectin/leptin ratio accompany the expansion and/or the dysfunction of adipose tissue, and have been associated with a multitude of adverse obesity-related outcomes [209, 210]. Interestingly, in an abundance of observational studies, corresponding changes of adipokine levels in relation to leukemia have been ascertained. Decreased adiponectin concentrations are observed in AML [211, 212], adult and childhood ALL [212, 213], in non-treated vs. treated CML [214], and CLL [215]. Leptin levels appear markedly increased in ALL [212] and decreased in AML [212, 216] and CLL [9]. Visfatin levels appear decreased in childhood acute leukemia and tend to normalize following HSC transplantation [217]. On the other hand, visfatin reduces AML blast proliferation, and its inhibition increases the sensitivity to chemotherapy, through the regulation of miR-IL-17 signaling via the PI3K/Akt pathway [218]. Resistin, a pro-inflammatory adipokine, is expressed in human AML and ALL cells [219], while its levels appear increased in childhood ALL [213].
Adiponectin, an anti-inflammatory adipokine, suppresses pro-inflammatory cytokine secretion by myeloid cells and T-lymphocytes and preserves HSC self-renewal and capacity to proliferate upon stimulation, while on the contrary, the absence of adiponectin receptor signaling may lead to sustained chronic cytokine-mediated HSC activation [220••], which in turn may promote the pre-leukemic state of clonal hematopoiesis [221]. AML cells express the leptin receptor whereby leptin binding increases the synthesis of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α [216, 222], while it exerts proliferative and anti-apoptotic effects [223, 224]. Conversely, in childhood ALL, a reduction of the expression of leptin receptor is observed on the surface of blast cells compared with healthy bone marrow cells [225], while remission after treatment is associated with an increased expression on circulating mononuclear cell populations [226].
A recent study has found that fasting inhibits the development of ALL but not AML in mouse models [227]. The authors have shown that that the development and maintenance of ALL is dependent on the decreased expression of the leptin-receptor (LEPR). They observed that fasting can inhibit the development of ALL by increasing the expression of LEPR and its downstream signaling through the protein PR/SET domain 1 (PRDM1). LEPR expression levels were also associated with the prognosis of pediatric patients with pre-B-ALL.
Another recent study has evaluated the role of pre-conditioning leptin levels in 524 patients with various hematologic malignancies in patients undergoing HSC transplantation [228]. Low levels of leptin were found to be an independent risk factor for an increased relapse risk. However, this marker did not show any correlation with overall mortality or non-relapse mortality. The effect was consistent in an independent validation cohort.
Collectively, perturbations of adipocytokine physiology are observed both in obesity-related adipose tissue dysfunction and leukemic disease, constituting a candidate pathogenetic link between the two conditions. Intervention targeting adipo(cyto)kine receptors or related signaling pathways may thus serve as targets for anti-leukemic therapy.
Bone Marrow Adiposity and Bone Marrow Microenvironment
Metabolic Characteristics of Bone Marrow Adipose Tissue and Leukemia
Bone marrow adipose tissue (BMAT) constitutes over 10% of total adipose tissue mass in lean individuals [229] and features distinct metabolic and secretory characteristics. Obesity, insulin resistance and dysglycemia are associated with BMAT expansion while the opposite effect is observed after treatment with metformin [230]. BMAT adipocytes are a source of adipokines such as leptin and adiponectin and likely exhibit a pattern of adipocytokine secretion which defers from that of visceral adipocytes; specifically, the mRNA levels of pro-inflammatory cytokines (TNFα, IL-1β) decrease in response to high-fat diet in mice, in contrast to their increase in peripheral adipose tissue [231]. Given their localization and proximity to HSCs, secretory signals deriving from BMAT, as well as their perturbations observed in obesity and dysmetabolism, may directly influence normal hematopoiesis and/or contribute to development of hematological disease, namely leukemias of myeloid origin [97]. BMAT expansion has been shown to negatively regulate normal hematopoiesis and is accompanied by a reduction of HSCs [232, 233]. BMAT expansion also promotes the pre-leukemic clonal hematopoiesis of HSCs harboring the DNMT3A mutations through IL-6 signaling [234]. Furthermore, existing evidence indicates that AML cells disrupt normal hematopoiesis by means of distorting BMAT function and impairing erythron-myeloid maturation, which is in turn restored after administration of PPARγ agonists [235]. Furthermore, it has been demonstrated that AML blasts induce a phosphorylation of hormone-sensitive lipase in BMAT and promote lipolysis, which in turn increases the abundance of FFAs and utilization by AML cells [236]. On the other hand, BMAT may hinder leukemic growth in T-ALL: injection of mice with human T-ALL blasts resulted in substantially lower infiltration of adipocyte-rich tail compared to thoracic vertebrae. Furthermore, blasts localized in the caudal compartment exhibited a different surface marker profile, lower proliferation rates, and suppressed metabolism which was however accompanied by the induction of resistance to vincristine [237]. These findings indicate that the dynamic interplay between BMAT and leukemic blasts likely results in differential effects on cell proliferation, metabolism, and chemotherapeutic resistance depending on cell origin.
In obesity, there is an abundance of nutrients that are stored in both the peripheral adipose tissue as well as in the bone marrow adipose niche. Τhe high concentrations of glucose, FFA, and AA could provide the energy supply for the proliferation and survival of the nearby leukemia cells [238]. Bone marrow provides the primary microenvironment for the development of leukemia. Mesenchymal stem cells from bone marrow biopsies of pediatric ALL patients have been found to highly express genes related to adipose tissue generation like CCAAT/enhancer-binding protein (CEBP) and PPARγ implying that the bone marrow is closely engaged with the adipose tissue [239].
In AML, leukemia cells have been shown to induce production of IGFBP1 from the adipose tissue to reduce insulin sensitivity and enhance their glucose uptake, favoring survival [137]. Furthermore, gut dysbiosis, lower serotonin, and incretin levels induced by the leukemic cells collectively inhibit insulin secretion; promoting thus cancer glucose uptake [137]. ALL cells display the Warburg effect where they prioritize glucose uptake that is dependent on GLUT1 receptor for their metabolic demands [240].
FFAs are an alternative source of energy for the proliferation and survival of leukemic cells [238]. In AML, adipocytes cultured together with blasts display upregulated expression of several enzymes involved in the metabolism and transport of fatty acids such as hormone-sensitive lipase, lipoprotein lipase, and fatty acid-binding protein-4 [236, 241]. In addition, leukemia cells can induce adipocytes to secrete FFAs that they can in turn use them in their advantage building elements of their cell membrane [242].
Aminoacids (AAs) are an essential metabolic source for all cells including blasts, and can be produced by adipocytes. ALL blasts do not express asparagine synthase which synthesizes the essential aminoacid asparagin, and are thus are susceptible to treatment to the drug L-asparaginase which inhibits asparagine synthase and further depletes this aminoacid rendering the blasts vulnerable [243]. Obesity can impair asparaginase efficacy in mice transplanted with ALL cells and without altering the plasma asparagine or glutamine levels [76]. The adipocytes residing in the bone marrow may contribute to therapeutic failure of L-asparaginase by supplying necessary AAs circumventing the deficiency of ALL cells [76].
The Role of Adipose-Derived Stem Cells
Adipose-derived stem cells (ASCs) are a kind of mesenchymal stem cells that may be detected in the vascular portion of the adipose tissue [244]. ASCs are a source of several molecules that are thought to promote tumor development such as IGF-1, transforming growth factor beta 1 (TGFβ1), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and IL-8 [245]. In the setting of ALL, human ASCs support the growth of cancer cell lines when co-delivered to xenografts, in a dose-dependent manner [246]. However, the role of ASCs is context dependent as they can exert pro- or anti-tumorigenic effects depending on the microenvironment [247]. ASCs can negatively affect anti-tumor immunity as they can inhibit the proliferation of NK cells the differentiation of dendritic cells into B- and T-lymphocytes [248].
Other Emerging Mechanisms
A number of other pathophysiological features, common between obesity and leukemic disease, constitute additional putative, albeit incompletely studied mechanisms which could pathogenetically link the two conditions.
The chronic low-grade inflammation that accompanies obesity and insulin resistance is associated with dysregulation of different functional aspects, which collectively result in a multidimensional immune dysfunction and, prominently, T-lymphocyte senescence [249,250,251]. This may lead to impaired immune surveillance and an increased propensity to malignancy, including leukemias. Besides, immune dysregulation is a cardinal feature of various types of leukemias [252,253,254,255], while effector T cell senescence may mediate resistance of AML cells to immunotherapy with checkpoint inhibitors [256]. Alterations of gut microbiota are featured in obesity and dysmetabolism as well as in various forms of leukemia, and their impact on the modulation of the immune system constitutes a field of active research in both conditions [257]. Perturbations in circadian clock gene expression have been implicated in the pathogenesis of obesity and insulin resistance [258,259,260] as well as in that of various leukemia types [261,262,263], although the role of circadian clock genes in the regulation of leukemogenesis has not been fully elucidated. It is still unclear whether these common pathogenetic features between obesity and leukemia are subject to therapeutic modulation.
Preventive and Therapeutic Perspectives
Preventive Measures (Diet, Bariatric Surgery, Physical Exercise)
The unequivocal epidemiological relationship between obesity and incidence of acute and chronic leukemias [31, 34, 43, 51, 74] as well as the numerous mechanisms linking the two conditions render the commonly implemented weight loss strategies of potential importance for the prevention of leukemia. Hypocaloric diets and increased physical activity constitute the mainstay of weight loss schemes, complemented by medical therapies and bariatric/metabolic surgery.
Data on the effects of caloric restriction-induced weight loss on leukemia risk are lacking, likely due to the limited feasibility of reduced weight maintenance in cohorts of adequate size in the long term. On the other hand, available evidence points towards an association between the qualitative composition of diet and leukemia risk. Based on the findings of a meta-analysis, increased maternal consumption of Mediterranean diet components, such as fruit, vegetables, legumes, and fish, has been associated with a lower risk of childhood leukemia, mainly ALL, while preconception folic acid and vitamin supplementation may also exert a protective effect. Consistent trends were observed on account of childhood dietary habits, together with a possible added risk by increased processed meat consumption [264]. A case-control study among pediatric patients aged 5–14 years has attributed a protective effect of milk and dairy consumption and a detrimental effect of added dietary lipids on ALL risk [265]. In contrast, adherence to a Western dietary pattern in adults has been associated with an increased CLL risk, independently of Rai stage [266]. Apart from the quantitative and qualitative dietary features, chrononutrition offers another dimension along which dietary interventions may impact on the manifestation and prognosis of various diseases, including malignancies. Although clinical evidence has been lacking to date, the implication of changes in clock gene expression in leukemias [263, 267] together with the dynamic impact of time restricted feeding on the pattern of gene expression in several tissues [268] may offer new perspectives for the prevention and management of leukemias.
Similarly to dietary interventions, there have been no observations on structured exercise programs to assess the effect of physical activity on leukemia risk. Nonetheless, evidence from observational studies indicates an inverse relationship between leisure-time physical activity and risk of myeloid leukemias, whereas no such association seems to exist with leukemias of lymphoid origin [269]. Accordingly, an adequate, compared with an insufficient, level of moderate-to-vigorous physical activity seems to be protective of the composite incidence of MDS and myeloid leukemias, although this observation is mainly driven by a reduction of MDS occurrence [270].
Besides, apart from prevention, a secondary intervention program implementing caloric restriction and increased physical activity to achieve a more than 20% caloric deficit significantly impacted ALL prognosis among individuals aged 10–21 years old, as indexed by significant reductions of minimal residual disease following chemotherapy compared with matched historical controls [271].
Obesity pharmacotherapy is a relatively newly developed field, precluding long-term observations on the effects of specific drug classes of leukemia occurrence. The beneficial effects of bariatric surgery, which constitutes the most effective currently available modality for prolonged weight loss and metabolic amelioration, on malignancy risk have been validated in long-term cohorts of operated patients. A reduction of incident total hematological malignancies was observed in participants of the Swedish obese subjects cohort [272]. However, the scarce evidence on leukemia-specific incidence is less compelling [273]. It should be noted that acquired copper deficiency which occasionally occurs as a complication of bariatric surgery [274, 275] is a secondary and potentially reversible cause of myelodysplastic bone marrow changes [276, 277], although the potential for malignant transformation of MDS which develops in the frame of copper deficiency is unclear.
Biomarkers
Several molecules which are altered in obesity and are associated with obesity-related complications [9, 35, 103, 278,279,280,281,282] seem to concomitantly play a role in the pathogenesis of certain leukemia types and/or exhibit prognostic attributes. Apart from their systemic hormonal actions, the expansion of BMAT in obesity and related functional adipocyte changes [283] may be hypothesized to at least partially mediate these associations through the modulation of the bone marrow paracrine microenvironment.
Levels of adipose tissue-derived acute phase reactants such as CRP, TNFα, or IL-6 constitute a striking paradigm; a higher CRP-to-albumin ratio at diagnosis has been associated with shorter overall survival in transplant-ineligible elderly patients with AML [284] and shorter treatment-free and overall survival newly in newly diagnosed CLL [285]. IL-6 induces pediatric AML cell resistance to chemotherapy-induced apoptosis in vitro, and accordingly, bone marrow IL-6 concentrations are negatively associated with event-free survival in pediatric AML [286]. Circulating IL-6 is elevated in ALL and CML, while in the latter case, higher levels are observed during the blast crisis phase of transformation towards AML [287]. TNFα may promote AML progression through activation of the NF-κB pathway [288]. Higher TNFα levels are also encountered in ALL cases and normalize after induction chemotherapy; furthermore, an incomplete suppression TNFα is associated with incomplete remission after induction chemotherapy [289].
Among leukemia subtypes, perturbations of various adipokines have been observed, the most thoroughly studied of which are leptin and adiponectin. Adipocytes secrete leptin proportionally to bodily fat stores and hence its levels strongly reflect the degree of adiposity [290]. Contrary to normal promyelocytes, promyelocytes in AML may express the leptin receptor, and accordingly be prone to leptin signaling-induced proliferative and anti-apoptotic effects [291]. Elevations of circulating leptin have been occasionally [292] but not universally [212, 293] reported in AML, as well as ALL [212]. Leptin levels are also increased in CLL and CML [294, 295] while they tend to normalize after successful imatinib treatment in CML [295]. Lower adiponectin levels are associated with adverse features in obesity such as visceral adiposity, adipose tissue inflammation, and dysmetabolism [12, 296, 297]. Accordingly, lower adiponectin concentrations have been ascertained among patients with MDS compared with matched controls [103, 279, 281, 298]. Similar observations have been made for adult and childhood AML [211, 212] while in the former case, adiponectin levels inversely correlate with the cellular burden of AML as indexed by LDH concentration and bone barrow blast proportion [212]. Adiponectin levels may also be lower in newly diagnosed CML [215] or prospectively rise after initiation of TKI treatment [214]. Similar observations have been made for adult ALL [212], although the evidence regarding CLL and childhood ALL is less convincing [279]. Serum visfatin, an adipokine positively associated with an adverse metabolic profile in obesity [27, 108, 299], exerts proliferative effects and induces resistance to chemotherapy in AML cells in vitro [218]. On the other hand, visfatin levels are lower in pediatric AL patients than controls and rise to control levels after HSC transplantation [217]. Resistin, another adipose tissue-derived biomarker with positive associations with visceral adiposity and IR [299,300,301], has been found to be higher in newly diagnosed and relapsed pediatric ALL compared with controls [302].
MicroRNAs (also miRs) are small, non-coding RNA molecules which can modulate gene expression, with a potential role in the pathogenesis of malignant disease [303]. Certain adipose tissue-derived microRNAs are expressed in the adipose tissue and may concomitantly play a role in the pathogenesis of leukemias being also potential biomarkers. miR-125b is highly expressed in the white adipose tissue, particularly in obesity [304]. The overexpression of miR-125b in mouse model induces B- or T-acute lymphocyte leukemia [303], while in humans, the homolog Hsa-miR-125b-1 is implicated in the translocations associated with B-ALL or AML [305]. Increased levels of miR-486-5p, which is also upregulated in obesity, are encountered in CML [306] and may attenuate CML-progenitor cell sensitivity to tyrosine kinase inhibitor therapy [307]. MiRNA-221 and -222 are overexpressed in the adipose tissue in obesity [308, 309] and may also modulate the sensitivity of leukemic cells to treatment in ALL [310], CML [311], and CLL [312]. Circulating miR-142-3p levels, which positively correlate with BMI, waist-to-hip ratio, and IR indices [313], are downregulated in AML and are associated with drug resistance [314].
Candidate Therapeutic Agents Targeting Oncogenic Pathways of Obesity and Insulin Resistance in Leukemia
Obesity and dysmetabolism-related perturbations in oncogenic pathways that play a role in leukemogenesis offer attractive prospects for the treatment of various leukemia types. In the crossroads of the two conditions, interventions aiming towards the loss of weight and metabolic amelioration could prove beneficial as preventive strategies. The increase of IR and reduction of insulin secretion induced by leukemic cells through various pathophysiological adaptive changes, including elevated expression of insulin-like growth factor binding protein 1 and suppression of incretin response, have been proposed as mechanisms promoting leukemic cell growth [137].
IGF-1 and insulin receptor expression has been ascertained in leukemic cells in AML [130, 315, 316], ALL [317, 318], CLL [113], and CML [319]. The activation of these receptors and the subsequent signal transduction through the PI3K-Akt-mTOR pathway play a central part in the leukemic cell growth and proliferation [320, 321]. This renders the drugs that target successive steps of these pathway potential candidates for leukemia treatment. Targeting of IGF1R signaling using pharmacological inhibitors (NT157/OSI-906), neutralizing antibodies, or Sorafenib induces anti-proliferative effects on ALL [317], AML [114], and CLL [113] cells in vitro, respectively. Idelalisib is a PI3Kδ inhibitor which is approved for the treatment of CLL, with demonstrated activity also against B ALL cells [322]. Furthermore, mTOR inhibitors Everolimus and Temsirolimus have shown promising results as adjunctive agents together with traditional drug therapy against leukemia in various settings in preclinical and early phase clinical trials [323,324,325,326,327,328].
Accordingly, repurposing of agents commonly used for the treatment of obesity-related metabolic disease offers useful perspectives for leukemia treatment as depicted in Table 4.
Metformin is a first-line agent for the treatment of type 2 DM. Metformin exhibits a multifaceted mechanism of action, predominantly through the activation of AMP-activated protein kinase (AMPK) [362]. AMPK-dependent intracellular pathways seem to play a pivotal role in oncogenesis, including leukemogenesis [363]. Various in vitro studies have demonstrated the anti-leukemic cellular properties of metformin; however, corresponding clinical data are to date lacking (Table 4).
PPARs partake in many aspects of cellular proliferation, apoptosis, and differentiation. Fibrates and thiazolidinediones (glitazones) constitute two widely used medication classes in hypertriglyceridemia and type 2 DM, respectively. Fibrates exert their actions through selective agonism of PPAR-α and lower triglycerides while increasing HDL levels [364], both typical components of “diabetic dyslipidemia.” Glitazones activate PPAR-γ and are used as insulin sensitizers. Both medication classes have demonstrated interesting anti-leukemic properties in preclinical studies (Table 4).
Aspirin and statins are used for the risk modification in patients with high cardiovascular risk or with established vascular disease. Furthermore, both classes seem to possess interesting anti-leukemic properties (Table 4). Most statin-related observations have been made in CLL cell lines, whereby statins exhibit anti-proliferative effects and synergism with purine analogues in vitro. Potential clinical benefits have also been noted, despite some concerns regarding a presumed reduction of the anti-tumor effects of agents targeting CD20, due to the induction of conformational CD20 changes by statin therapy [365].
The Challenges of the COVID-19 Pandemic
The COVID-19 pandemic brought about an unprecedented crisis affects virtually every aspect of clinical care. Patients with hematological malignancies were particularly affected, due to the complexity of the management of their illness necessitating either adherence to a strict therapeutic schedule or a chronic proximity to healthcare services at various levels [366]. Already early in the course of the pandemic, the presence of particularly active, hematological malignancies was recognized as a factor associated with frequent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) acquisition and a more severe disease course [367]. Patients with leukemia are at a particularly high risk of COVID-19 due to factors associated with leukemic disease itself or its treatment (among others, leukopenia and lymphopenia, impaired cellular and humoral immunity, hypercoagulability, organ dysfunction) [368]. CLL patients treated with anti-CD20 agents (i.e., Rituximab) constitute a unique patient collective with regards to COVID-19; apart from the hypogammaglobulinemia associated with CLL, treatment-induced B-lymphocyte depletion further impairs the effective immunity development after receiving standard vaccination schemes [369], while it is also associated with impaired viral clearance in case of SARS-CoV-2 infection [370], occasionally with prolonged viral shedding [371]. Furthermore, blood product transfusions which are an inseparable component of leukemia management received a significant negative impact particularly in the early stages of the pandemic due to initial concerns regarding virus transmissibility as well as blood donation volume reductions and blood bank reserve depletions [366].
The necessity for timely therapy, including stem cell transplantation where indicated, following leukemia diagnosis and adherence to (often long term) treatment schemes should be weighed against the acute detrimental effects of treatment on the immune status of leukemia patients and subsequent risks of COVID-19 acquisition and adverse course, particularly around the peak of pandemic waves. Given the impact of adequate therapy on leukemia prognosis, there is little room for compromise with respect to delays or modification on treatment schedule. In peak pandemic periods, risk minimization strategies should be pursued [368], together with meticulous COVID-19 diagnostic screening even in asymptomatic individuals. Additionally, rationalization of transfusion strategies on a case-by-case basis is necessary in the face of blood product shortages. Lastly, although no uniformly accepted strategy exists for the passive immunization of leukemia patients against COVID-19, a more meticulous vaccination schedule could be chosen for selected patient groups, since repeat or multiple vaccinations have been shown to increase seroconversion rates in patients with impaired humoral immunity in the frame of B cell neoplastic disorders and their therapies [369, 372].
Perspectives and Conclusions
Understanding the association between excess body weight and leukemia may present important implications for the prevention and treatment. Obesity represents an interesting risk factor for leukemia, being among the only known risk factors that could be prevented or modified while current research is mainly focused on the development of novel and expensive treatments for leukemia.
Emphasis on leukemia prevention could prevent several cases of leukemia. In the era of precision medicine, an important approach would be to perform large, multicentric, well-designed prospective studies to investigate whether obesity is a predisposing factor for the development of leukemia. As obesity is a modifiable factor, weight loss, healthy diet, and physical exercise may decrease the risk of cancers including leukemia [373••, 374,375,376]. Moreover, pharmacological interventions, repurposing drugs used for cardiometabolic comorbidities, and bariatric surgery may be highly recommended for leukemia and obesity-related cancer prevention [376,377,378,379].
Furthermore, the majority of studies evaluating the association between obesity and leukemia have used BMI as an index of obesity. Nonetheless, BMI presents several limitations when used as a marker of obesity, such as the lack of information regarding adipose distribution or visceral fat obesity [19, 63]. Other more reliable markers, such as waist circumference, waist-to-hip ratio, dual X-ray absorptiometry determinations, or magnetic resonance imaging, may be used. In terms of the pathogenetic mechanisms connecting obesity with leukemia, wider basic and translational research is required to further elucidate the complex molecular networks through which excess body weight influences the disease course providing potential therapeutic options.
Epidemiological evidence suggests a connection between obesity and leukemia. In addition, obesity is associated with worse outcomes and increased mortality in leukemic patients.
Abbreviations
- AAs:
-
Aminoacids
- AGA:
-
Appropriate for gestational age
- ALL:
-
Acute lymphocytic leukemia
- AML:
-
Acute myeloid leukemia
- AMPK:
-
AMP-activated protein kinase
- APL:
-
Acute promyelocytic leukemia
- ASCs:
-
Adipose-derived stem cells
- ATRA:
-
ALL trans retinoic acid
- BMAT:
-
Bone marrow adipose tissue
- BMI:
-
Body mass index
- BW:
-
Birthweight
- CCSS:
-
Childhood Cancer Survivor Study
- CEBP:
-
CCAAT/enhancer-binding protein
- CDC:
-
Centers for Disease Control and Prevention
- CEBP:
-
CCAAT/enhancer-binding protein
- CI:
-
Confidence intervals
- CLL:
-
Chronic lymphocytic leukemia
- CML:
-
Chronic myeloid leukemia
- CNS:
-
Central nervous system
- CRP:
-
C-reactive protein
- CRT:
-
Cranial radiation therapy
- CVD:
-
Cardiovascular disease
- DAMPs:
-
Damage-associated molecular patterns
- DM:
-
Diabetes mellitus
- DS:
-
Differentiation syndrome
- EFS:
-
Event-free survival
- ER:
-
Estrogen receptor
- EWAS:
-
Epigenome-Wide Association Study
- FFAs:
-
Free fatty acids
- GAL-9:
-
Galectin-9
- GH:
-
Growth hormone
- GWAS:
-
Genome-Wide Association Study
- HDL:
-
High-density lipoprotein
- HGF:
-
Hepatocyte growth factor
- HR:
-
Hazards ratio
- HSCs:
-
Hematopoietic stem cells
- IARC:
-
International Agency for Research on Cancer
- IFN:
-
Interferon
- IGF:
-
Insulin-like growth factors
- IGFBP:
-
Insulin-like growth factor binding protein
- IL:
-
Interleukin
- IL-1RAP:
-
IL-1 receptor accessory protein
- InsR:
-
Insulin receptor
- IR:
-
Insulin resistance
- IRS:
-
Insulin receptor substrates
- LDL:
-
Low-density lipoprotein
- LEPR:
-
Leptin receptor
- LGA:
-
Large for gestational age
- LIC:
-
Leukemia initiating cells
- MDS:
-
Myelodysplastic syndromes
- MiRs:
-
MicroRNAs
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PAMPs:
-
Pathogen-associated molecular patterns
- PFS:
-
Progression-free survival
- PI3K:
-
Phosphoinositide 3-kinase
- PPARα/γ:
-
Peroxisome proliferator-activated receptor α or γ
- RCT:
-
Randomized controlled trials
- ROS:
-
Reactive oxygen species
- RR:
-
Relative risk
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SCF:
-
Stem cell factor
- SJLIFE:
-
St. Jude Lifetime Cohort
- S1P:
-
Sphingosine-1-phosphate
- TGFβ1:
-
Transforming growth factor beta 1
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-alpha
- VEGF:
-
Vascular endothelial growth factor
- vs:
-
Versus
- WHO:
-
World Health Organization
- y.o.:
-
Years old
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO Classification of Tumours, Revised 4th Edition, Volume 2. International Agency for Research on Cancer; 2017.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:96. https://doi.org/10.1186/s13045-019-0783-9.
Bispo JAB, Pinheiro PS, Kobetz EK. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb Perspect Med. 2020:10. https://doi.org/10.1101/cshperspect.a034819.
Du M, Chen W, Liu K, Wang L, Hu Y, Mao Y, et al. The Global burden of leukemia and its attributable factors in 204 countries and territories: Findings from the global burden of disease 2019 study and projections to 2030. J Oncol. 2022;2022:1612702. https://doi.org/10.1155/2022/1612702.
Dalamaga M, Petridou E, Cook FE, Trichopoulos D. Risk factors for myelodysplastic syndromes: A case-control study in Greece. Cancer Causes Control. 2002;13:603–8. https://doi.org/10.1023/a:1019573319803.
Hao T, Li-Talley M, Buck A, Chen W. An emerging trend of rapid increase of leukemia but not all cancers in the aging population in the United States. Sci Rep. 2019;9:12070. https://doi.org/10.1038/s41598-019-48445-1.
Dalamaga M, Lekka A, Karmaniolas K, Stathopoulou E, Dionyssiou-Asteriou A. Is thyroid autoimmunity a risk factor for developing primary myelodysplastic syndrome? Cancer Causes Control. 2008;19:371–8. https://doi.org/10.1007/s10552-007-9096-3.
Dalamaga M, Crotty BH, Fargnoli J, Papadavid E, Lekka A, Triantafilli M, et al. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: A case-control study in Greece. Cancer Causes Control. 2010;21:1451–9. https://doi.org/10.1007/s10552-010-9573-y.
Petridou E, Dalamaga M, Mentis A, Skalkidou A, Moustaki M, Karpathios T, et al. Evidence on the infectious etiology of childhood leukemia: The role of low herd immunity (Greece). Cancer Causes Control. 2001;12:645–52. https://doi.org/10.1023/a:1011255825887.
Lichtman MA. Obesity and the risk for a hematological malignancy: Leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083–101. https://doi.org/10.1634/theoncologist.2010-0206.
Hroussalas G, Kassi E, Dalamaga M, Delimaris I, Zachari A, Dionyssiou-Asteriou A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas. 2008;59:339–49. https://doi.org/10.1016/j.maturitas.2008.03.012.
Kassi E, Dalamaga M, Faviou E, Hroussalas G, Kazanis K, Nounopoulos C, et al. Circulating oxidized LDL levels, current smoking and obesity in postmenopausal women. Atherosclerosis. 2009;205:279–83. https://doi.org/10.1016/j.atherosclerosis.2008.11.006.
Paroutoglou K, Papadavid E, Christodoulatos GS, Dalamaga M. Deciphering the association between psoriasis and obesity: Current evidence and treatment considerations. Curr Obes Rep. 2020;9:165–78. https://doi.org/10.1007/s13679-020-00380-3.
Koliaki C, Liatis S, Dalamaga M, Kokkinos A. The implication of gut hormones in the regulation of energy homeostasis and their role in the pathophysiology of obesity. Curr Obes Rep. 2020;9:255–71. https://doi.org/10.1007/s13679-020-00396-9.
Stratigou T, Dalamaga M, Antonakos G, Marinou I, Vogiatzakis E, Christodoulatos GS, et al. Hyperirisinemia is independently associated with subclinical hypothyroidism: Correlations with cardiometabolic biomarkers and risk factors. Endocrine. 2018;61:83–93. https://doi.org/10.1007/s12020-018-1550-3.
Fogarasi A, Gonzalez K, Dalamaga M, Magkos F. The impact of the rate of weight loss on body composition and metabolism. Curr Obes Rep. 2022;11:33–44. https://doi.org/10.1007/s13679-022-00470-4.
WHO. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed on March 25, 2023.
Liu J, Tsilingiris D, Dalamaga M. The non-linear relationship between muscle mass and BMI calls into question the use of BMI as a major criterion for eligibility for bariatric surgery. Metabol Open. 2022;13:100164. https://doi.org/10.1016/j.metop.2022.100164.
Simati S, Kokkinos A, Dalamaga M, Argyrakopoulou G. Obesity paradox: Fact or fiction? Curr Obes Rep. 2023;12:75–85. https://doi.org/10.1007/s13679-023-00497-1.
Afshin A, Reitsma MB, Murray CJL. Health effects of overweight and obesity in 195 countries. N Engl J Med. 2017;377:1496–7. https://doi.org/10.1056/NEJMc1710026.
Karampela I, Vallianou N, Magkos F, Apovian CM, Dalamaga M. Obesity, hypovitaminosis D, and COVID-19: The Bermuda triangle in public health. Curr Obes Rep. 2022;11:116–25. https://doi.org/10.1007/s13679-022-00471-3.
Cardel MI, Atkinson MA, Taveras EM, Holm JC, Kelly AS. Obesity treatment among adolescents: A review of current evidence and future directions. JAMA Pediatr. 2020;174:609–17. https://doi.org/10.1001/jamapediatrics.2020.0085.
Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin Cancer Biol. 2021;73:356–76. https://doi.org/10.1016/j.semcancer.2021.05.008.
Tsilingiris D, Liatis S, Dalamaga M, Kokkinos A. The fight against obesity escalates: New Drugs on the horizon and metabolic implications. Curr Obes Rep. 2020;9:136–49. https://doi.org/10.1007/s13679-020-00378-x.
Tsigalou C, Vallianou N, Dalamaga M. Autoantibody production in obesity: Is there evidence for a link between obesity and autoimmunity? Curr Obes Rep. 2020;9:245–54. https://doi.org/10.1007/s13679-020-00397-8.
Dalamaga M, Papadavid E, Basios G, Vaggopoulos V, Rigopoulos D, Kassanos D, et al. Ovarian SAHA syndrome is associated with a more insulin-resistant profile and represents an independent risk factor for glucose abnormalities in women with polycystic ovary syndrome: A prospective controlled study. J Am Acad Dermatol. 2013;69:922–30. https://doi.org/10.1016/j.jaad.2013.09.014.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. https://doi.org/10.1056/NEJMsr1606602.
Larsson SC, Spyrou N, Mantzoros CS. Body fatness associations with cancer: Evidence from recent epidemiological studies and future directions. Metabolism. 2022;137:155326. https://doi.org/10.1016/j.metabol.2022.155326.
Castillo JJ, Reagan JL, Ingham RR, Furman M, Dalia S, Merhi B, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: A meta-analysis of prospective cohort studies. Leuk Res. 2012;36:868–75. https://doi.org/10.1016/j.leukres.2011.12.020.
Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21. https://doi.org/10.1002/ijc.23176.
Lichtman MA. Obesity and the risk of chronic myelogenous leukemia: Is this another example of the neoplastic effects of increased body fat? Leukemia. 2012;26:183–4. https://doi.org/10.1038/leu.2011.190.
Teras LR, Patel AV, Carter BD, Rees-Punia E, McCullough ML, Gapstur SM. Anthropometric factors and risk of myeloid leukaemias and myelodysplastic syndromes: A prospective study and meta-analysis. Br J Haematol. 2019;186:243–54. https://doi.org/10.1111/bjh.15904.
Poynter JN, Richardson M, Blair CK, Roesler MA, Hirsch BA, Nguyen P, et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134–40. https://doi.org/10.1016/j.canep.2015.12.005.
Dalamaga M, Karmaniolas K, Lekka A, Antonakos G, Thrasyvoulides A, Papadavid E, et al. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Dis Markers. 2010;29:55–61. https://doi.org/10.3233/dma-2010-0726.
Bhaskaran K, Douglas I, Forbes H, dos Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–65. https://doi.org/10.1016/s0140-6736(14)60892-8.
Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: Estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13:72. https://doi.org/10.1186/s13045-020-00908-z.
Huang J, Chan SC, Ngai CH, Lok V, Zhang L, Lucero-Prisno DE 3rd, et al. Disease Burden, risk factors, and trends of leukaemia: A global analysis. Front Oncol. 2022;12:904292. https://doi.org/10.3389/fonc.2022.904292.
Ahmed M, Mäkinen VP, Lumsden A, Boyle T, Mulugeta A, Lee SH, et al. Metabolic profile predicts incident cancer: A large-scale population study in the UK Biobank. Metabolism. 2023;138:155342. https://doi.org/10.1016/j.metabol.2022.155342.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. https://doi.org/10.1056/NEJMoa021423.
Amankwah EK, Saenz AM, Hale GA, Brown PA. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: A systematic review and meta-analysis. Leuk Lymphoma. 2016;57:1140–8. https://doi.org/10.3109/10428194.2015.1076815.
Jeddi R, Ghédira H, Mnif S, Gouider E, Fenaux P, Meddeb B. High body mass index is an independent predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leuk Res. 2010;34:545–7. https://doi.org/10.1016/j.leukres.2009.09.017.
Strom SS, Yamamura Y, Kantarijian HM, Cortes-Franco JE. Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 2009;18:1501–6. https://doi.org/10.1158/1055-9965.Epi-09-0028.
Wong O, Harris F, Yiying W, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: Analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol. 2009;55:340–52. https://doi.org/10.1016/j.yrtph.2009.08.007.
Chiu BC, Gapstur SM, Greenland P, Wang R, Dyer A. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2348–54. https://doi.org/10.1158/1055-9965.Epi-06-0007.
Kasim K, Levallois P, Abdous B, Auger P, Johnson KC. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Causes Control. 2005;16:489–500. https://doi.org/10.1007/s10552-004-7115-1.
Ross JA, Parker E, Blair CK, Cerhan JR, Folsom AR. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev. 2004;13:1810–3.
Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11:1661–4. https://doi.org/10.1038/sj.leu.2400783.
Ma X, Lim U, Park Y, Mayne ST, Wang R, Hartge P, et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169:1492–9. https://doi.org/10.1093/aje/kwp074.
Reagan JL, Ingham RR II, Dalia S, Furman M, Merhi B, Nemr S, et al. Association between obesity/overweight and leukemia: A meta-analysis of prospective cohort studies. Blood. 2011;118:3588. https://doi.org/10.1182/blood.V118.21.3588.3588.
Mazzarella L, Botteri E, Matthews A, Gatti E, Di Salvatore D, Bagnardi V, et al. Obesity is a risk factor for acute promyelocytic leukemia: Evidence from population and cross-sectional studies and correlation with FLT3 mutations and polyunsaturated fatty acid metabolism. Haematologica. 2020;105:1559–66. https://doi.org/10.3324/haematol.2019.223925.
Milne E, Royle JA, de Klerk NH, Blair E, Bailey H, Cole C, et al. Fetal growth and risk of childhood acute lymphoblastic leukemia: Results from an Australian case-control study. Am J Epidemiol. 2009;170:221–8. https://doi.org/10.1093/aje/kwp117.
Caughey RW, Michels KB. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–70. https://doi.org/10.1002/ijc.24225.
Huang T, Ducore JM. Children and adolescents with ALL are taller than expected at diagnosis. J Pediatr Hematol Oncol. 2014;36:16–21. https://doi.org/10.1097/MPH.0b013e31829bcb10.
Schraw JM, Henson AT, Scheurer ME, Forman MR. The associations of height-for-age, weight-for-age, and weight-for-height with pediatric acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2017;39:376–81. https://doi.org/10.1097/mph.0000000000000874.
Sprehe MR, Barahmani N, Cao Y, Wang T, Forman MR, Bondy M, et al. Comparison of birth weight corrected for gestational age and birth weight alone in prediction of development of childhood leukemia and central nervous system tumors. Pediatr Blood Cancer. 2010;54:242–9. https://doi.org/10.1002/pbc.22308.
Stacy SL, Buchanich JM, Ma ZQ, Mair C, Robertson L, Sharma RK, et al. Maternal obesity, birth size, and risk of childhood cancer development. Am J Epidemiol. 2019;188:1503–11. https://doi.org/10.1093/aje/kwz118.
•• Jiménez-Hernández E, Fajardo-Gutiérrez A, Núñez-Enriquez JC, Martín-Trejo JA, Espinoza-Hernández LE, Flores-Lujano J, et al. A greater birthweight increases the risk of acute leukemias in Mexican children-experience from the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia (MIGICCL). Cancer Med. 2018;7:1528–36. https://doi.org/10.1002/cam4.1414. This is an interesting original research study among Mexican children that demonstrated a relationship between greater birth weight and increased risk of acute leukemia.
Tran LT, Lai HTM, Koriyama C, Uwatoko F, Akiba S. The association between high birth weight and the risks of childhood CNS tumors and leukemia: An analysis of a US case-control study in an epidemiological database. BMC Cancer. 2017;17:687. https://doi.org/10.1186/s12885-017-3681-y.
Hjalgrim LL, Westergaard T, Rostgaard K, Schmiegelow K, Melbye M, Hjalgrim H, et al. Birth weight as a risk factor for childhood leukemia: A meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158:724–35. https://doi.org/10.1093/aje/kwg210.
Marley AR, Ryder JR, Turcotte LM, Spector LG. Maternal obesity and acute lymphoblastic leukemia risk in offspring: A summary of trends, epidemiological evidence, and possible biological mechanisms. Leuk Res. 2022;121:106924. https://doi.org/10.1016/j.leukres.2022.106924.
Marley AR, Domingues A, Ghosh T, Turcotte LM, Spector LG. Maternal body mass index, diabetes, and gestational weight gain and risk for pediatric cancer in offspring: A systematic review and meta-analysis. JNCI Cancer Spectr. 2022:6. https://doi.org/10.1093/jncics/pkac020.
Dalamaga M, Stratigou T, Tsilingiris D. Body composition and fat depot assessment: Going supersonic to improve cardiometabolic outcomes. Pol. Arch Intern Med. 2022:132. https://doi.org/10.20452/pamw.16362.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001.
Foster KL, Kern KD, Chambers TM, Lupo PJ, Kamdar KY, Scheurer ME, et al. Weight trends in a multiethnic cohort of pediatric acute lymphoblastic leukemia survivors: A longitudinal analysis. PLoS One. 2019;14:e0217932. https://doi.org/10.1371/journal.pone.0217932.
Garmey EG, Liu Q, Sklar CA, Meacham LR, Mertens AC, Stovall MA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–45. https://doi.org/10.1200/jco.2008.16.3527.
Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in pediatric ALL survivors: A meta-analysis. Pediatrics. 2014;133:e704–15. https://doi.org/10.1542/peds.2013-3332.
• Richard MA, Brown AL, Belmont JW, Scheurer ME, Arroyo VM, Foster KL, et al. Genetic variation in the body mass index of adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Cancer. 2021;127:310–8. https://doi.org/10.1002/cncr.33258. In this multicentric study, adult survivors of childhood ALL have genetic heritability for BMI similar to that observed in the general population, providing evidence that treatment with cranial radiation therapy can modify the effect of genetic variants on adult BMI in childhood ALL survivors.
Green DM, Cox CL, Zhu L, Krull KR, Srivastava DK, Stovall M, et al. Risk factors for obesity in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30:246–55. https://doi.org/10.1200/jco.2010.34.4267.
Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–6. https://doi.org/10.1038/nature20784.
•• Lupo PJ, Brown AL, Arroyo VM, Kamdar KY, Belmont JW, Scheurer ME, et al. DNA methylation and obesity in survivors of pediatric acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Genes Chromosomes Cancer. 2019;58:52–9. https://doi.org/10.1002/gcc.22701. A very interesting original study showing that BMI-DNA methylation loci differ between ALL survivors, who received only chemotherapy when compared to those that received cranial radiotherapy as well.
Pramanik R, Sheng X, Ichihara B, Heisterkamp N, Mittelman SD. Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res. 2013;37:503–9. https://doi.org/10.1016/j.leukres.2012.12.013.
Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–74. https://doi.org/10.1158/0008-5472.Can-09-0800.
Dushnicky MJ, Nazarali S, Mir A, Portwine C, Samaan MC. Is there a causal relationship between childhood obesity and acute lymphoblastic leukemia? A review. Cancers (Basel). 2020:12. https://doi.org/10.3390/cancers12113082.
Núñez-Enríquez JC, Gil-Hernández AE, Jiménez-Hernández E, Fajardo-Gutiérrez A, Medina-Sansón A, Flores-Lujano J, et al. Overweight and obesity as predictors of early mortality in Mexican children with acute lymphoblastic leukemia: A multicenter cohort study. BMC Cancer. 2019;19:708. https://doi.org/10.1186/s12885-019-5878-8.
Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI, et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res. 2013;73:2998–3006. https://doi.org/10.1158/0008-5472.Can-12-4402.
Sheng X, Tucci J, Parmentier JH, Ji L, Behan JW, Heisterkamp N, et al. Adipocytes cause leukemia cell resistance to daunorubicin via oxidative stress response. Oncotarget. 2016;7:73147–59. https://doi.org/10.18632/oncotarget.12246.
Lee M, Hamilton JAG, Talekar GR, Ross AJ, Michael L, Rupji M, et al. Obesity-induced galectin-9 is a therapeutic target in B-cell acute lymphoblastic leukemia. Nat Commun. 2022;13:1157. https://doi.org/10.1038/s41467-022-28839-y.
Sheng X, Mittelman SD. The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front Pediatr. 2014;2:53. https://doi.org/10.3389/fped.2014.00053.
Sheng X, Parmentier JH, Tucci J, Pei H, Cortez-Toledo O, Dieli-Conwright CM, et al. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol Cancer Res. 2017;15:1704–13. https://doi.org/10.1158/1541-7786.Mcr-17-0338.
• Shimony S, Flamand Y, Valtis YK, Place AE, Silverman LB, Vrooman LM, et al. Effect of BMI on toxicities and survival among adolescents and young adults treated on DFCI Consortium ALL Trials. Blood Adv. 2023;7:5234–45. https://doi.org/10.1182/bloodadvances.2023009976. Amid adolescent and young adults with acute lymphoblastic leukemia treated on DFCI Consortium ALL regimens, elevated BMI was associated with increased toxicity and decreased overall survival.
Liu Q, Major B, Le-Rademacher J, Al-Kali AA, Alkhateeb H, Begna K, et al. The impact of obesity on the outcomes of adult patients with acute lymphoblastic leukemia - a single center retrospective study. Blood Lymphat Cancer. 2021;11:1–9. https://doi.org/10.2147/blctt.S269748.
Medeiros BC, Othus M, Estey EH, Fang M, Appelbaum FR. Impact of body-mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica. 2012;97:1401–4. https://doi.org/10.3324/haematol.2011.056390.
Dhakal P, Lyden E, Lee A, Michalski J, Al-Kadhimi ZS, Maness LJ, et al. Effects of obesity on overall survival of adults with acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020;20:e131–e6. https://doi.org/10.1016/j.clml.2019.11.001.
Li S, Chen L, Jin W, Ma X, Ma Y, Dong F, et al. Influence of body mass index on incidence and prognosis of acute myeloid leukemia and acute promyelocytic leukemia: A meta-analysis. Sci Rep. 2017;7:17998. https://doi.org/10.1038/s41598-017-18278-x.
Baillargeon J, Langevin AM, Lewis M, Estrada J, Mullins J, Pitney A, et al. Obesity and survival in a cohort of predominantly Hispanic children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2006;28:575–8. https://doi.org/10.1097/01.mph.0000212985.33941.d8.
Ethier MC, Alexander S, Abla O, Green G, Lam R, Sung L. Association between obesity at diagnosis and weight change during induction and survival in pediatric acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53:1677–81. https://doi.org/10.3109/10428194.2012.664843.
Aldhafiri FK, McColl JH, Reilly JJ. Prognostic significance of being overweight and obese at diagnosis in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2014;36:234–6. https://doi.org/10.1097/mph.0000000000000056.
Orgel E, Genkinger JM, Aggarwal D, Sung L, Nieder M, Ladas EJ. Association of body mass index and survival in pediatric leukemia: A meta-analysis. Am J Clin Nutr. 2016;103:808–17. https://doi.org/10.3945/ajcn.115.124586.
Saenz AM, Stapleton S, Hernandez RG, Hale GA, Goldenberg NA, Schwartz S, et al. Body mass index at pediatric leukemia diagnosis and the risks of relapse and mortality: Findings from a single institution and meta-analysis. J Obes. 2018;2018:7048078. https://doi.org/10.1155/2018/7048078.
Gelelete CB, Pereira SH, Azevedo AM, Thiago LS, Mundim M, Land MG, et al. Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity (Silver Spring). 2011;19:1908–11. https://doi.org/10.1038/oby.2011.195.
Núñez-Enríquez JC, Bárcenas-López DA, Hidalgo-Miranda A, Jiménez-Hernández E, Bekker-Méndez VC, Flores-Lujano J, et al. Gene expression profiling of acute lymphoblastic leukemia in children with very early relapse. Arch Med Res. 2016;47:644–55. https://doi.org/10.1016/j.arcmed.2016.12.005.
Gaggini M, Carli F, Gastaldelli A. The color of fat and its central role in the development and progression of metabolic diseases. Horm Mol Biol. Clin Investig. 2017:31. https://doi.org/10.1515/hmbci-2017-0060.
Cinti S. Pink Adipocytes. Trends Endocrinol Metab. 2018;29:651–66. https://doi.org/10.1016/j.tem.2018.05.007.
Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: Inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019;8:434–57. https://doi.org/10.1007/s13679-019-00360-2.
Li Y, Cao S, Gaculenko A, Zhan Y, Bozec A, Chen X. Distinct metabolism of bone marrow adipocytes and their role in bone metastasis. Front Endocrinol (Lausanne). 2022;13:902033. https://doi.org/10.3389/fendo.2022.902033.
Piotrowska K, Tarnowski M. Bone marrow adipocytes-role in physiology and various nutritional conditions in human and animal models. Nutrients. 2021:13. https://doi.org/10.3390/nu13051412.
Martinez LM, Guzman ML. Understanding the interaction between leukaemia stem cells and their microenvironment to improve therapeutic approaches. Br J Pharmacol. 2023; https://doi.org/10.1111/bph.16162.
Pottosin I, Olivas-Aguirre M, Dobrovinskaya O. In vitro simulation of the acute lymphoblastic leukemia niche: A critical view on the optimal approximation for drug testing. J Leukoc Biol. 2023;114:21–41. https://doi.org/10.1093/jleuko/qiad039.
Karamanakos G, Kokkinos A, Dalamaga M, Liatis S. Highlighting the role of obesity and insulin resistance in type 1 diabetes and its associated cardiometabolic complications. Curr Obes Rep. 2022;11:180–202. https://doi.org/10.1007/s13679-022-00477-x.
Tsilingiris D, Dalamaga M, Liu J. SARS-CoV-2 adipose tissue infection and hyperglycemia: A further step towards the understanding of severe COVID-19. Metabol Open. 2022;13:100163. https://doi.org/10.1016/j.metop.2022.100163.
Tsilingiris D, Tzeravini E, Koliaki C, Dalamaga M, Kokkinos A. The role of mitochondrial adaptation and metabolic flexibility in the pathophysiology of obesity and insulin resistance: An updated overview. Curr Obes Rep. 2021;10:191–213. https://doi.org/10.1007/s13679-021-00434-0.
Dalamaga M, Karmaniolas K, Chamberland J, Nikolaidou A, Lekka A, Dionyssiou-Asteriou A, et al. Higher fetuin-A, lower adiponectin and free leptin levels mediate effects of excess body weight on insulin resistance and risk for myelodysplastic syndrome. Metabolism. 2013;62:1830–9. https://doi.org/10.1016/j.metabol.2013.09.007.
Leitner BP, Siebel S, Akingbesote ND, Zhang X, Perry RJ. Insulin and cancer: A tangled web. Biochem J. 2022;479:583–607. https://doi.org/10.1042/bcj20210134.
Søndergaard CS, Esquivel PN, Dalamaga M, Magkos F. Use of antihyperglycemic drugs and risk of cancer in patients with diabetes. Curr Oncol Rep. 2023;25:29–40. https://doi.org/10.1007/s11912-022-01344-7.
Stratigou T, Muscogiuri G, Kotopouli M, Antonakos G, Christodoulatos GS, Karampela I, et al. Lower circulating omentin-1 is independently linked to subclinical hypothyroidism reflecting cardiometabolic risk: An observational case-control and interventional, longitudinal study. Panminerva Med. 2022;64:452–64. https://doi.org/10.23736/s0031-0808.22.04701-2.
Christodoulatos GS, Antonakos G, Karampela I, Psallida S, Stratigou T, Vallianou N, et al. Circulating Omentin-1 as a biomarker at the intersection of postmenopausal breast cancer occurrence and cardiometabolic risk: An observational cross-sectional study. Biomolecules. 2021:11. https://doi.org/10.3390/biom11111609.
Spyrou N, Avgerinos KI, Mantzoros CS, Dalamaga M. Classic and novel adipocytokines at the intersection of obesity and cancer: Diagnostic and therapeutic strategies. Curr Obes Rep. 2018;7:260–75. https://doi.org/10.1007/s13679-018-0318-7.
Kasuga M, Kahn CR, Hedo JA, Van Obberghen E, Yamada KM. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981;78:6917–21. https://doi.org/10.1073/pnas.78.11.6917.
Saiya-Cork K, Collins R, Parkin B, Ouillette P, Kuizon E, Kujawski L, et al. A pathobiological role of the insulin receptor in chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:2679–92. https://doi.org/10.1158/1078-0432.Ccr-10-2058.
Chen PM, Kwan SH, Hwang TS, Chiang BN, Chou CK. Insulin receptors on leukemia and lymphoma cells. Blood. 1983;62:251–5.
Medyouf H, Gusscott S, Wang H, Tseng JC, Wai C, Nemirovsky O, et al. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J Exp Med. 2011;208:1809–22. https://doi.org/10.1084/jem.20110121.
Yaktapour N, Übelhart R, Schüler J, Aumann K, Dierks C, Burger M, et al. Insulin-like growth factor-1 receptor (IGF1R) as a novel target in chronic lymphocytic leukemia. Blood. 2013;122:1621–33. https://doi.org/10.1182/blood-2013-02-484386.
Chapuis N, Tamburini J, Cornillet-Lefebvre P, Gillot L, Bardet V, Willems L, et al. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: Therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica. 2010;95:415–23. https://doi.org/10.3324/haematol.2009.010785.
Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: Are we making headway? Front Oncol. 2022;12:819128. https://doi.org/10.3389/fonc.2022.819128.
Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal. 2009;7:14. https://doi.org/10.1186/1478-811x-7-14.
Machado-Neto JA, Fenerich BA, Rodrigues Alves APN, Fernandes JC, Scopim-Ribeiro R, Coelho-Silva JL, et al. Insulin substrate receptor (IRS) proteins in normal and malignant hematopoiesis. Clinics (Sao Paulo). 2018;73:e566s. https://doi.org/10.6061/clinics/2018/e566s.
Traina F, Carvalheira JB, Saad MJ, Costa FF, Saad ST. BCR-ABL binds to IRS-1 and IRS-1 phosphorylation is inhibited by imatinib in K562 cells. FEBS Lett. 2003;535:17–22. https://doi.org/10.1016/s0014-5793(02)03845-0.
Yin T, Keller SR, Quelle FW, Witthuhn BA, Tsang ML, Lienhard GE, et al. Interleukin-9 induces tyrosine phosphorylation of insulin receptor substrate-1 via JAK tyrosine kinases. J Biol Chem. 1995;270:20497–502. https://doi.org/10.1074/jbc.270.35.20497.
Savage SL, Eide CA, Concannon KF, Bottomly D, Wilmot B, McWeeney SK, et al. Activating mutations of insulin receptor substrate 2 (IRS2) in patients with tyrosine kinase inhibitor-refractory chronic myeloid leukemia. Blood. 2015;126:2461. https://doi.org/10.1182/blood.V126.23.2461.2461.
Zhao H, Liu F, Jia R, Chang H, Li H, Miao M, et al. MiR-570 inhibits cell proliferation and glucose metabolism by targeting IRS1 and IRS2 in human chronic myelogenous leukemia. Iran J Basic Med Sci. 2017;20:481–8. https://doi.org/10.22038/ijbms.2017.8671.
de Melo CP, Machado-Neto JA, Eide CA, Savage SL, Scopim-Ribeiro R, da Silva Souza Duarte A, et al. IRS2 silencing increases apoptosis and potentiates the effects of ruxolitinib in JAK2V617F-positive myeloproliferative neoplasms. Oncotarget. 2016;7:6948–59. https://doi.org/10.18632/oncotarget.6851.
Chang YC, Lin HC, Chiang YH, Chen CG, Huang L, Wang WT, et al. Targeted next-generation sequencing identified novel mutations in triple-negative myeloproliferative neoplasms. Med Oncol. 2017;34:83. https://doi.org/10.1007/s12032-017-0944-z.
Fernandes JC, Rodrigues Alves APN, Machado-Neto JA, Scopim-Ribeiro R, Fenerich BA, da Silva FB, et al. IRS1/β-catenin axis is activated and induces MYC expression in acute lymphoblastic leukemia cells. J Cell Biochem. 2017;118:1774–81. https://doi.org/10.1002/jcb.25845.
Juric D, Lacayo NJ, Ramsey MC, Racevskis J, Wiernik PH, Rowe JM, et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25:1341–9. https://doi.org/10.1200/jco.2006.09.3534.
Ye W, Jiang Z, Lu X, Ren X, Deng M, Lin S, et al. GZD824 suppresses the growth of human B cell precursor acute lymphoblastic leukemia cells by inhibiting the SRC kinase and PI3K/AKT pathways. Oncotarget. 2017;8:87002–15. https://doi.org/10.18632/oncotarget.10881.
Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, et al. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol. 1999;19:7203–15. https://doi.org/10.1128/mcb.19.10.7203.
Guo S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–t23. https://doi.org/10.1530/joe-13-0327.
Leclerc GM, Leclerc GJ, Fu G, Barredo JC. AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Signal. 2010;5:15. https://doi.org/10.1186/1750-2187-5-15.
Nepstad I, Hatfield KJ, Grønningsæter IS, Aasebø E, Hernandez-Valladares M, Hagen KM, et al. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct Target Ther. 2019;4:20. https://doi.org/10.1038/s41392-019-0050-0.
Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T, et al. The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia. 2007;21:886–96. https://doi.org/10.1038/sj.leu.2404643.
Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–85. https://doi.org/10.1002/cncr.20071.
Pan J, Chen C, Jin Y, Fuentes-Mattei E, Velazquez-Tores G, Benito JM, et al. Differential impact of structurally different anti-diabetic drugs on proliferation and chemosensitivity of acute lymphoblastic leukemia cells. Cell Cycle. 2012;11:2314–26. https://doi.org/10.4161/cc.20770.
Abe S, Funato T, Takahashi S, Yokoyama H, Yamamoto J, Tomiya Y, et al. Increased expression of insulin-like growth factor i is associated with Ara-C resistance in leukemia. Tohoku J Exp Med. 2006;209:217–28. https://doi.org/10.1620/tjem.209.217.
Dawczynski K, Kauf E, Schlenvoigt D, Gruhn B, Fuchs D, Zintl F. Elevated serum insulin-like growth factor binding protein-2 is associated with a high relapse risk after hematopoietic stem cell transplantation in childhood AML. Bone Marrow Transplant. 2006;37:589–94. https://doi.org/10.1038/sj.bmt.1705281.
Karmali R, Borgia JA, Larson ML, Shammo JM, Basu S, Venugopal P. Impact of circulating members of the insulin-like growth factor receptor (IGF-1R) axis on outcomes in acute myeloid leukemia. J Clin Oncol. 2014;32:7069. https://doi.org/10.1200/jco.2014.32.15_suppl.7069.
Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M, et al. Subversion of systemic glucose metabolism as a mechanism to support the growth of leukemia cells. Cancer Cell. 2018;34:659–73.e6. https://doi.org/10.1016/j.ccell.2018.08.016.
Vekic J, Stefanovic A, Zeljkovic A. Obesity and dyslipidemia: A review of current evidence. Curr Obes Rep. 2023; https://doi.org/10.1007/s13679-023-00518-z.
Lempesis IG, Apple SJ, Duarte G, Palaiodimos L, Kalaitzopoulos DR, Dalamaga M, et al. Cardiometabolic effects of SGLT2 inhibitors on polycystic ovary syndrome. Diabetes Metab Res Rev. 2023:e3682. https://doi.org/10.1002/dmrr.3682.
Lempesis IG, Varrias D, Sagris M, Attaran RR, Altin ES, Bakoyiannis C, et al. Obesity and peripheral artery disease: Current evidence and controversies. Curr Obes Rep. 2023:1–16. https://doi.org/10.1007/s13679-023-00510-7.
Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control. 2011;22:291–9. https://doi.org/10.1007/s10552-010-9697-0.
Spiegel RJ, Schaefer EJ, Magrath IT, Edwards BK. Plasma lipid alterations in leukemia and lymphoma. Am J Med. 1982;72:775–82. https://doi.org/10.1016/0002-9343(82)90543-5.
Nagel G, Stocks T, Späth D, Hjartåker A, Lindkvist B, Hallmans G, et al. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can). Ann Hematol. 2012;91:1519–31. https://doi.org/10.1007/s00277-012-1489-z.
Advani AS, McDonough S, Copelan E, Willman C, Mulford DA, List AF, et al. SWOG0919: a Phase 2 study of idarubicin and cytarabine in combination with pravastatin for relapsed acute myeloid leukaemia. Br J Haematol. 2014;167:233–7. https://doi.org/10.1111/bjh.13035.
Kornblau SM, Banker DE, Stirewalt D, Shen D, Lemker E, Verstovsek S, et al. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose Ara-C: A phase 1 study. Blood. 2007;109:2999–3006. https://doi.org/10.1182/blood-2006-08-044446.
Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–21. https://doi.org/10.1038/sj.ijo.0802853.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43. https://doi.org/10.1097/MED.0b013e3283444b09.
Hierons SJ, Abbas K, Sobczak AIS, Cerone M, Smith TK, Ajjan RA, et al. Changes in plasma free fatty acids in obese patients before and after bariatric surgery highlight alterations in lipid metabolism. Sci Rep. 2022;12:15337. https://doi.org/10.1038/s41598-022-19657-9.
Fugmann M, Uhl O, Hellmuth C, Hetterich H, Kammer NN, Ferrari U, et al. Differences in the serum nonesterified Fatty Acid profile of young women associated with a recent history of gestational diabetes and overweight/obesity. PLoS One. 2015;10:e0128001. https://doi.org/10.1371/journal.pone.0128001.
Milligan G, Shimpukade B, Ulven T, Hudson BD. Complex pharmacology of free fatty acid receptors. Chem Rev. 2017;117:67–110. https://doi.org/10.1021/acs.chemrev.6b00056.
Khalid A, Siddiqui AJ, Huang JH, Shamsi T, Musharraf SG. Alteration of serum free fatty acids are indicators for progression of pre-leukaemia diseases to leukaemia. Sci Rep. 2018;8:14883. https://doi.org/10.1038/s41598-018-33224-1.
Tucci J, Chen T, Margulis K, Orgel E, Paszkiewicz RL, Cohen MD, et al. Adipocytes provide fatty acids to acute lymphoblastic leukemia cells. Front Oncol. 2021;11:665763. https://doi.org/10.3389/fonc.2021.665763.
Culp-Hill R, Stevens BM, Jones CL, Pei S, Dzieciatkowska M, Minhajuddin M, et al. Therapy-resistant acute myeloid leukemia stem cells are resensitized to venetoclax + azacitidine by targeting fatty acid desaturases 1 and 2. Metabolites. 2023:13. https://doi.org/10.3390/metabo13040467.
Nomura DK, Long JZ, Niessen S, Hoover HS, Ng S-W, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. https://doi.org/10.1016/j.cell.2009.11.027.
Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: A potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91:6379–83. https://doi.org/10.1073/pnas.91.14.6379.
Birmann BM, Giovannucci E, Rosner B, Anderson KC, Colditz GA. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2007;16:1474–8. https://doi.org/10.1158/1055-9965.Epi-07-0143.
Stevenson CE, Takabe K, Nagahashi M, Milstien S, Spiegel S. Targeting sphingosine-1-phosphate in hematologic malignancies. Anticancer Agents Med Chem. 2011;11:794–8. https://doi.org/10.2174/187152011797655122.
Sun J, Lou Y, Zhu J, Shen H, Zhou D, Zhu L, et al. Hypertriglyceridemia in newly diagnosed acute promyelocytic leukemia. Front Oncol. 2020;10:577796. https://doi.org/10.3389/fonc.2020.577796.
Usman H, Rashid R, Ameer F, Iqbal A, Zaid M, Hasnain S, et al. Revisiting the dyslipidemia associated with acute leukemia. Clin Chim Acta. 2015;444:43–9. https://doi.org/10.1016/j.cca.2015.01.038.
Scribano D, Baroni S, Pagano L, Zuppi C, Leone G, Giardina B. Return to normal values of lipid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica. 1996;81:343–5.
Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998;83:379–84.
Mulas MF, Abete C, Pulisci D, Pani A, Massidda B, Dessì S, et al. Cholesterol esters as growth regulators of lymphocytic leukaemia cells. Cell Prolif. 2011;44:360–71. https://doi.org/10.1111/j.1365-2184.2011.00758.x.
Bai S, Wang H, Shao R, Fu B, Lu S, Wang J, et al. Lipid profile as a novel prognostic predictor for patients with acute myeloid leukemia. Front Oncol. 2023;13:950732. https://doi.org/10.3389/fonc.2023.950732.
Hirano T. Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb. 2018;25:771–82. https://doi.org/10.5551/jat.RV17023.
Gill JM, Brown JC, Bedford D, Wright DM, Cooney J, Hughes DA, et al. Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle-aged subjects. Atherosclerosis. 2004;176:49–56. https://doi.org/10.1016/j.atherosclerosis.2004.04.022.
Wu S, Li S, Jin P, Zhang Y, Chen L, Jin W, et al. Interplay between hypertriglyceridemia and acute promyelocytic leukemia mediated by the cooperation of peroxisome proliferator-activated receptor-α with the PML/RAR α fusion protein on super-enhancers. Haematologica. 2022;107:2589–600. https://doi.org/10.3324/haematol.2021.280147.
Argyrakopoulou G, Dalamaga M, Spyrou N, Kokkinos A. Gender differences in obesity-related cancers. Curr Obes Rep. 2021;10:100–15. https://doi.org/10.1007/s13679-021-00426-0.
Papavasileiou G, Tsilingiris D, Spyrou N, Vallianou NG, Karampela I, Magkos F, et al. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin Cancer Biol. 2023;91:70–98. https://doi.org/10.1016/j.semcancer.2023.03.002.
Trakakis E, Papadavid E, Dalamaga M, Koumaki D, Stavrianeas N, Rigopoulos D, et al. Prevalence of non classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Greek women with acne: A hospital-based cross-sectional study. J Eur Acad Dermatol Venereol. 2013;27:1448–51. https://doi.org/10.1111/j.1468-3083.2012.04613.x.
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. https://doi.org/10.1182/blood-2005-06-2508.
Forsythe A, Breland T, Majumdar S, Elkin TD, Johnson D, Megason G. Gender differences in incidence rates of childhood B-precursor acute lymphocytic leukemia in Mississippi. J Pediatr Oncol Nurs. 2010;27:164–7. https://doi.org/10.1177/1043454209357919.
Roma A, Spagnuolo PA. Estrogen receptors alpha and beta in acute myeloid leukemia. Cancers (Basel). 2020:12. https://doi.org/10.3390/cancers12040907.
Hillisch A, Peters O, Kosemund D, Müller G, Walter A, Schneider B, et al. Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol Endocrinol. 2004;18:1599–609. https://doi.org/10.1210/me.2004-0050.
Griffiths EA, Gore SD, Hooker CM, Mohammad HP, McDevitt MA, Smith BD, et al. Epigenetic differences in cytogenetically normal versus abnormal acute myeloid leukemia. Epigenetics. 2010;5:590–600. https://doi.org/10.4161/epi.5.7.12558.
Parl FF, Crooke PS, Plummer WD Jr, Dupont WD. Genomic-epidemiologic evidence that estrogens promote breast cancer development. Cancer Epidemiol Biomarkers Prev. 2018;27:899–907. https://doi.org/10.1158/1055-9965.Epi-17-1174.
Klein HU, Ruckert C, Kohlmann A, Bullinger L, Thiede C, Haferlach T, et al. Quantitative comparison of microarray experiments with published leukemia related gene expression signatures. BMC Bioinform. 2009;10:422. https://doi.org/10.1186/1471-2105-10-422.
Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, van Waalwijk B, van Doorn-Khosrovani S, Boer JM, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–28. https://doi.org/10.1056/NEJMoa040465.
Rota S-G, Roma A, Dude I, Ma C, Stevens R, MacEachern J, et al. Estrogen receptor β is a novel target in acute myeloid leukemia. Mol Cancer Ther. 2017;16:2618–26. https://doi.org/10.1158/1535-7163.Mct-17-0292.
Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126:1549–64. https://doi.org/10.1161/CIRCRESAHA.119.315896.
Luciano M, Krenn PW, Horejs-Hoeck J. The cytokine network in acute myeloid leukemia. Front Immunol. 2022;13:1000996. https://doi.org/10.3389/fimmu.2022.1000996.
Pérez-Figueroa E, Sánchez-Cuaxospa M, Martínez-Soto KA, Sánchez-Zauco N, Medina-Sansón A, Jiménez-Hernández E, et al. Strong inflammatory response and Th1-polarization profile in children with acute lymphoblastic leukemia without apparent infection. Oncol Rep. 2016;35:2699–706. https://doi.org/10.3892/or.2016.4657.
Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26:6738–49. https://doi.org/10.1038/sj.onc.1210758.
Pasupuleti SK, Ramdas B, Burns SS, Palam LR, Kanumuri R, Kumar R, et al. Obesity-induced inflammation exacerbates clonal hematopoiesis. J Clin Invest. 2023:133. https://doi.org/10.1172/jci163968.
Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105. https://doi.org/10.1016/j.yjmcc.2021.07.004.
Barreyro L, Chlon TM, Starczynowski DT. Chronic immune response dysregulation in MDS pathogenesis. Blood. 2018;132:1553–60. https://doi.org/10.1182/blood-2018-03-784116.
Carey A, Edwards DK, Eide CA, Newell L, Traer E, Medeiros BC, et al. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18:3204–18. https://doi.org/10.1016/j.celrep.2017.03.018.
Paracatu LC, Schuettpelz LG. Contribution of aberrant toll like receptor signaling to the pathogenesis of myelodysplastic syndromes. Front Immunol. 2020;11:1236.
Stubbins RJ, Platzbecker U, Karsan A. Inflammation and myeloid malignancy: Quenching the flame. Blood, J Am Soc Hematol. 2022;140:1067–74.
Hormaechea-Agulla D, Matatall KA, Le DT, Kain B, Long X, Kus P, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell. 2021;28:1428–42.e6. https://doi.org/10.1016/j.stem.2021.03.002.
Lasry A, Nadorp B, Fornerod M, Nicolet D, Wu H, Walker CJ, et al. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat Cancer. 2023;4:27–42.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44. https://doi.org/10.1089/met.2015.0095.
Peluso M, Russo V, Mello T, Galli A. Oxidative stress and DNA damage in chronic disease and environmental studies. Int J Mol Sci. 2020:21. https://doi.org/10.3390/ijms21186936.
Udensi UK, Tchounwou PB. Dual effect of oxidative stress on leukemia cancer induction and treatment. J Exp Clin Cancer Res. 2014;33:106. https://doi.org/10.1186/s13046-014-0106-5.
Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch Med Sci. 2013;9:191–200. https://doi.org/10.5114/aoms.2013.33181.
Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. https://doi.org/10.1530/joe-13-0339.
Clemente-Suárez VJ, Redondo-Flórez L, Beltrán-Velasco AI, Martín-Rodríguez A, Martínez-Guardado I, Navarro-Jiménez E, et al. The role of adipokines in health and disease. Biomedicines. 2023:11. https://doi.org/10.3390/biomedicines11051290.
Krenn PW, Montanez E, Costell M, Fässler R. Integrins, anchors and signal transducers of hematopoietic stem cells during development and in adulthood. Curr Top Dev Biol. 2022;149:203–61. https://doi.org/10.1016/bs.ctdb.2022.02.009.
Hemmati S, Haque T, Gritsman K. Inflammatory signaling pathways in preleukemic and leukemic stem cells. Front Oncol. 2017;7:265. https://doi.org/10.3389/fonc.2017.00265.
Camacho V, McClearn V, Patel S, Welner RS. Regulation of normal and leukemic stem cells through cytokine signaling and the microenvironment. Int J Hematol. 2017;105:566–77. https://doi.org/10.1007/s12185-017-2184-6.
Pietras EM. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130:1693–8. https://doi.org/10.1182/blood-2017-06-780882.
Ahmed HA, Maklad AM, Khaled SA, Elyamany A. Interleukin-27 and interleukin-35 in de novo acute myeloid leukemia: Expression and significance as biological markers. J Blood Med. 2019;10:341–9. https://doi.org/10.2147/jbm.S221301.
Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Bañas H, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: Survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–91. https://doi.org/10.1016/j.cyto.2012.12.023.
Tao M, Li B, Nayini J, Andrews CB, Huang RW, Devemy E, et al. SCF, IL-1beta, IL-1ra and GM-CSF in the bone marrow and serum of normal individuals and of AML and CML patients. Cytokine. 2000;12:699–707. https://doi.org/10.1006/cyto.2000.0666.
Elbaz O, Shaltout A. Implication of granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-3 (IL-3) in children with acute myeloid leukaemia (AML); malignancy. Hematology. 2001;5:383–8.
Beauchemin V, Villeneuve L, Rodriguez-Cimadevilla JC, Rajotte D, Kenney JS, Clark SC, et al. Interleukin-6 production by the blast cells of acute myeloblastic leukemia: Regulation by endogenous interleukin-1 and biological implications. J Cell Physiol. 1991;148:353–61. https://doi.org/10.1002/jcp.1041480305.
Villatoro A, Cuminetti V, Bernal A, Torroja C, Cossío I, Benguría A, et al. Endogenous IL-1 receptor antagonist restricts healthy and malignant myeloproliferation. Nat Commun. 2023;14:12. https://doi.org/10.1038/s41467-022-35700-9.
Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, von Palffy S, et al. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci U S A. 2015;112:10786–91. https://doi.org/10.1073/pnas.1422749112.
Trad R, Warda W, Alcazer V, Neto da Rocha M, Berceanu A, Nicod C, et al. Chimeric antigen receptor T-cells targeting IL-1RAP: A promising new cellular immunotherapy to treat acute myeloid leukemia. J Immunother Cancer. 2022:10. https://doi.org/10.1136/jitc-2021-004222.
Hasegawa T, Harada M, Kimura S, Take A, Yoshida I, Horimi H, et al. Four years' experience with the Omniscience prosthetic valve. Kyobu Geka. 1986;39:292–5.
Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. https://doi.org/10.1080/21623945.2017.1402151.
Petridou E, Mantzoros CS, Dessypris N, Dikalioti SK, Trichopoulos D. Adiponectin in relation to childhood myeloblastic leukaemia. Br J Cancer. 2006;94:156–60. https://doi.org/10.1038/sj.bjc.6602896.
Aref S, Ibrahim L, Azmy E, Al AR. Impact of serum adiponectin and leptin levels in acute leukemia. Hematology. 2013;18:198–203. https://doi.org/10.1179/1607845412y.0000000059.
El-Baz HA, Mosa TE, Elabd EM, Ramadan A, Elharoun AS, Elmorsy EA, et al. Serum adiponectin and resistin levels in de novo and relapsed acute lymphoblastic leukemia children patients. Iran J Public Health. 2013;42:504–10.
Fitter S, Vandyke K, Schultz CG, White D, Hughes TP, Zannettino AC. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: a mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab. 2010;95:3763–7. https://doi.org/10.1210/jc.2010-0086.
Avcu F, Ural AU, Yilmaz MI, Bingol N, Nevruz O, Caglar K. Association of plasma adiponectin concentrations with chronic lymphocytic leukemia and myeloproliferative diseases. Int J Hematol. 2006;83:254–8. https://doi.org/10.1532/ijh97.Na0411.
Bruserud Ø, Huang TS, Glenjen N, Gjertsen BT, Foss B. Leptin in human acute myelogenous leukemia: Studies of in vivo levels and in vitro effects on native functional leukemia blasts. Haematologica. 2002;87:584–95.
Skoczen S, Tomasik PJ, Gozdzik J, Fijorek K, Krasowska-Kwiecien A, Wiecha O, et al. Visfatin concentrations in children with leukemia before and after stem cell transplantation. Exp Hematol. 2014;42:252–60. https://doi.org/10.1016/j.exphem.2013.12.006.
Hui Z, Liu Z, He A, Chen Y, Zhang P, Lei B, et al. Visfatin promotes the malignancy of human acute myeloid leukemia cells via regulation of IL-17. Eur J Pharmacol. 2019;853:103–10. https://doi.org/10.1016/j.ejphar.2019.03.016.
Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–35. https://doi.org/10.1016/j.bbrc.2003.09.093.
•• Meacham CE, Jeffery EC, Burgess RJ, Sivakumar CD, Arora MA, Stanley AM, et al. Adiponectin receptors sustain haematopoietic stem cells throughout adulthood by protecting them from inflammation. Nat Cell Biol. 2022;24:697–707. https://doi.org/10.1038/s41556-022-00909-9. In this original research study, it was found that adiponectin receptors sustain hematopoietic stem cells throughout adulthood by protecting them from inflammation.
Demerdash Y, Kain B, Essers MAG, King KY. Yin and Yang: The dual effects of interferons on hematopoiesis. Exp Hematol. 2021;96:1–12. https://doi.org/10.1016/j.exphem.2021.02.002.
Mouzaki A, Panagoulias I, Dervilli Z, Zolota V, Spadidea P, Rodi M, et al. Expression patterns of leptin receptor (OB-R) isoforms and direct in vitro effects of recombinant leptin on OB-R, leptin expression and cytokine secretion by human hematopoietic malignant cells. Cytokine. 2009;48:203–11. https://doi.org/10.1016/j.cyto.2009.07.006.
Kim JY, Park HK, Yoon JS, Kim SJ, Kim ES, Song SH, et al. Molecular mechanisms of cellular proliferation in acute myelogenous leukemia by leptin. Oncol Rep. 2010;23:1369–74. https://doi.org/10.3892/or_00000773.
Foss B, Mentzoni L, Bruserud O. Effects of vascular endothelial growth factor on acute myelogenous leukemia blasts. J Hematother Stem Cell Res. 2001;10:81–93. https://doi.org/10.1089/152581601750098291.
Gorska E, Popko K, Wasik M. Leptin receptor in childhood acute leukemias. Adv Exp Med Biol. 2013;756:155–61. https://doi.org/10.1007/978-94-007-4549-0_20.
Wex H, Ponelis E, Wex T, Dressendörfer R, Mittler U, Vorwerk P. Plasma leptin and leptin receptor expression in childhood acute lymphoblastic leukemia. Int J Hematol. 2002;76:446–52. https://doi.org/10.1007/bf02982810.
Lu Z, Xie J, Wu G, Shen J, Collins R, Chen W, et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat Med. 2017;23:79–90. https://doi.org/10.1038/nm.4252.
Schwarzbich M-A, Dai H, Kordelas L, Beelen DW, Radujkovic A, Müller-Tidow C, et al. Pre-transplant serum leptin levels and relapse of acute myeloid leukemia after allogeneic transplantation. Int J Mol Sci. 2022;23:2337.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. https://doi.org/10.1016/j.cmet.2014.06.003.
Bornstein S, Moschetta M, Kawano Y, Sacco A, Huynh D, Brooks D, et al. Metformin affects cortical bone mass and marrow adiposity in diet-induced obesity in male mice. Endocrinology. 2017;158:3369–85. https://doi.org/10.1210/en.2017-00299.
Tencerova M, Figeac F, Ditzel N, Taipaleenmäki H, Nielsen TK, Kassem M. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res. 2018;33:1154–65. https://doi.org/10.1002/jbmr.3408.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. https://doi.org/10.1038/nature08099.
Zhu RJ, Wu MQ, Li ZJ, Zhang Y, Liu KY. Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol. 2013;97:58–72. https://doi.org/10.1007/s12185-012-1233-4.
Zioni N, Bercovich AA, Chapal-Ilani N, Bacharach T, Rappoport N, Solomon A, et al. Inflammatory signals from fatty bone marrow support DNMT3A driven clonal hematopoiesis. Nat Commun. 2023;14:2070. https://doi.org/10.1038/s41467-023-36906-1.
Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19:1336–47. https://doi.org/10.1038/ncb3625.
Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–32. https://doi.org/10.1182/blood-2016-08-734798.
Cahu X, Calvo J, Poglio S, Prade N, Colsch B, Arcangeli ML, et al. Bone marrow sites differently imprint dormancy and chemoresistance to T-cell acute lymphoblastic leukemia. Blood Adv. 2017;1:1760–72. https://doi.org/10.1182/bloodadvances.2017004960.
Orgel E, Sea JL, Mittelman SD. Mechanisms by which obesity impacts survival from acute lymphoblastic leukemia. J Natl Cancer Inst Monogr. 2019;2019:152–6. https://doi.org/10.1093/jncimonographs/lgz020.
Vicente López Á, Vázquez García MN, Melen GJ, Entrena Martínez A, Cubillo Moreno I, García-Castro J, et al. Mesenchymal stromal cells derived from the bone marrow of acute lymphoblastic leukemia patients show altered BMP4 production: Correlations with the course of disease. PLoS One. 2014;9:e84496. https://doi.org/10.1371/journal.pone.0084496.
Liu T, Kishton RJ, Macintyre AN, Gerriets VA, Xiang H, Liu X, et al. Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis. 2014;5:e1470. https://doi.org/10.1038/cddis.2014.431.
van't Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ, et al. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91:56–63.
Tucci J, Sheng X, Mittelman SD. Acute lymphoblastic leukemia cells stimulate adipocyte lipolysis and utilize adipocyte-derived free-fatty acids for proliferation. Cancer Res. 2014;74:4339.
Zhou R, Liang T, Li T, Huang J, Chen C. Possible mechanism of metabolic and drug resistance with L-asparaginase therapy in childhood leukaemia. Front Oncol. 2023;13:1070069. https://doi.org/10.3389/fonc.2023.1070069.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. https://doi.org/10.1091/mbc.e02-02-0105.
Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: Cancer metabolism. J Clin Oncol. 2016;34:4277–83. https://doi.org/10.1200/jco.2016.67.9712.
Lee MW, Park YJ, Kim DS, Park HJ, Jung HL, Lee JW, et al. Human adipose tissue stem cells promote the growth of acute lymphoblastic leukemia cells in NOD/SCID mice. Stem Cell Rev Rep. 2018;14:451–60. https://doi.org/10.1007/s12015-018-9806-0.
Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: Current status and challenges. Leukemia. 2019;33:597–611. https://doi.org/10.1038/s41375-018-0373-9.
Wang YC, Chen RF, Brandacher G, Lee WPA, Kuo YR. The suppression effect of dendritic cells maturation by adipose-derived stem cells through TGF-β1 related pathway. Exp Cell Res. 2018;370:708–17. https://doi.org/10.1016/j.yexcr.2018.07.037.
Daryabor G, Kabelitz D, Kalantar K. An update on immune dysregulation in obesity-related insulin resistance. Scand J Immunol. 2019;89:e12747. https://doi.org/10.1111/sji.12747.
Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. https://doi.org/10.3945/an.115.010207.
Sbierski-Kind J, Goldeck D, Buchmann N, Spranger J, Volk HD, Steinhagen-Thiessen E, et al. T cell phenotypes associated with insulin resistance: Results from the Berlin Aging Study II. Immun Ageing. 2020;17:40. https://doi.org/10.1186/s12979-020-00211-y.
Barrett AJ. Acute myeloid leukaemia and the immune system: Implications for immunotherapy. Br J Haematol. 2020;188:147–58. https://doi.org/10.1111/bjh.16310.
Pastorczak A, Domka K, Fidyt K, Poprzeczko M, Firczuk M. Mechanisms of immune evasion in acute lymphoblastic leukemia. Cancers (Basel). 2021:13. https://doi.org/10.3390/cancers13071536.
Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–81. https://doi.org/10.1182/blood-2015-03-567388.
Koldej RM, Prabahran A, Tan CW, Ng AP, Davis MJ, Ritchie DS. Dissection of the bone marrow microenvironment in hairy cell leukaemia identifies prognostic tumour and immune related biomarkers. Sci Rep. 2021;11:19056. https://doi.org/10.1038/s41598-021-98536-1.
Rutella S, Vadakekolathu J, Mazziotta F, Reeder S, Yau TO, Mukhopadhyay R, et al. Immune dysfunction signatures predict outcomes and define checkpoint blockade-unresponsive microenvironments in acute myeloid leukemia. J Clin Invest. 2022:132. https://doi.org/10.1172/jci159579.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. https://doi.org/10.1038/s41422-020-0332-7.
Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–81. https://doi.org/10.1016/j.cub.2013.01.048.
Škrlec I, Talapko J, Džijan S, Cesar V, Lazić N, Lepeduš H. The association between circadian clock gene polymorphisms and metabolic syndrome: A systematic review and meta-analysis. Biology (Basel). 2021:11. https://doi.org/10.3390/biology11010020.
Sardon Puig L, Pillon NJ, Näslund E, Krook A, Zierath JR. Influence of obesity, weight loss, and free fatty acids on skeletal muscle clock gene expression. Am J Physiol Endocrinol Metab. 2020;318:E1–e10. https://doi.org/10.1152/ajpendo.00289.2019.
Hanoun M, Eisele L, Suzuki M, Greally JM, Hüttmann A, Aydin S, et al. Epigenetic silencing of the circadian clock gene CRY1 is associated with an indolent clinical course in chronic lymphocytic leukemia. PLoS One. 2012;7:e34347. https://doi.org/10.1371/journal.pone.0034347.
Sun CM, Huang SF, Zeng JM, Liu DB, Xiao Q, Tian WJ, et al. Per2 inhibits k562 leukemia cell growth in vitro and in vivo through cell cycle arrest and apoptosis induction. Pathol Oncol Res. 2010;16:403–11. https://doi.org/10.1007/s12253-009-9227-0.
Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, et al. Core circadian clock genes regulate leukemia stem cells in AML. Cell. 2016;165:303–16. https://doi.org/10.1016/j.cell.2016.03.015.
Dessypris N, Karalexi MA, Ntouvelis E, Diamantaras AA, Papadakis V, Baka M, et al. Association of maternal and index child's diet with subsequent leukemia risk: A systematic review and meta analysis. Cancer Epidemiol. 2017;47:64–75. https://doi.org/10.1016/j.canep.2017.01.003.
Diamantaras AA, Dessypris N, Sergentanis TN, Ntouvelis E, Athanasiadou-Piperopoulou F, Baka M, et al. Nutrition in early life and risk of childhood leukemia: A case-control study in Greece. Cancer Causes Control. 2013;24:117–24. https://doi.org/10.1007/s10552-012-0097-5.
Solans M, Castelló A, Benavente Y, Marcos-Gragera R, Amiano P, Gracia-Lavedan E, et al. Adherence to the Western, Prudent, and Mediterranean dietary patterns and chronic lymphocytic leukemia in the MCC-Spain study. Haematologica. 2018;103:1881–8. https://doi.org/10.3324/haematol.2018.192526.
Gery S, Koeffler HP. Per2 is a C/EBP target gene implicated in myeloid leukemia. Integr Cancer Ther. 2009;8:317–20. https://doi.org/10.1177/1534735409352084.
Deota S, Lin T, Chaix A, Williams A, Le H, Calligaro H, et al. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 2023;35:150–65.e4. https://doi.org/10.1016/j.cmet.2022.12.006.
Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA. Intern Med. 2016;176:816–25. https://doi.org/10.1001/jamainternmed.2016.1548.
Rees-Punia E, Patel AV, Fallon EA, Gapstur SM, Teras LR. Physical activity, sitting time, and risk of myelodysplastic syndromes, acute myeloid leukemia, and other myeloid malignancies. Cancer Epidemiol Biomarkers Prev. 2019;28:1489–94. https://doi.org/10.1158/1055-9965.Epi-19-0232.
Orgel E, Framson C, Buxton R, Kim J, Li G, Tucci J, et al. Caloric and nutrient restriction to augment chemotherapy efficacy for acute lymphoblastic leukemia: The IDEAL trial. Blood Adv. 2021;5:1853–61. https://doi.org/10.1182/bloodadvances.2020004018.
Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. https://doi.org/10.1016/s1470-2045(09)70159-7.
Tao W, Santoni G, von Euler-Chelpin M, Ljung R, Lynge E, Pukkala E, et al. Cancer risk after bariatric surgery in a cohort study from the five nordic countries. Obes Surg. 2020;30:3761–7. https://doi.org/10.1007/s11695-020-04751-6.
Kumar P, Hamza N, Madhok B, De Alwis N, Sharma M, Miras AD, et al. Copper deficiency after gastric bypass for morbid obesity: A systematic review. Obes Surg. 2016;26:1335–42. https://doi.org/10.1007/s11695-016-2162-8.
Argyrakopoulou G, Konstantinidou SK, Dalamaga M, Kokkinos A. Nutritional deficiencies before and after bariatric surgery: Prevention and treatment. Curr Nutr Rep. 2022;11:95–101. https://doi.org/10.1007/s13668-022-00400-9.
Luo T, Zurko J, Astle J, Shah NN. Mimicking myelodysplastic syndrome: Importance of differential diagnosis. Case Rep Hematol. 2021;2021:9661765. https://doi.org/10.1155/2021/9661765.
D'Angelo G. Copper deficiency mimicking myelodysplastic syndrome. Blood Res. 2016;51:217–9. https://doi.org/10.5045/br.2016.51.4.217.
Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. https://doi.org/10.1016/j.metabol.2018.12.006.
Dalamaga M, Christodoulatos GS. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Investig. 2015;23:5–20. https://doi.org/10.1515/hmbci-2015-0016.
Dalamaga M, Polyzos SA, Karmaniolas K, Chamberland J, Lekka A, Triantafilli M, et al. Fetuin-A levels and free leptin index are reduced in patients with chronic lymphocytic leukemia: A hospital-based case-control study. Leuk Lymphoma. 2016;57:577–84. https://doi.org/10.3109/10428194.2015.1075523.
Dalamaga M, Karmaniolas K, Nikolaidou A, Chamberland J, Hsi A, Dionyssiou-Asteriou A, et al. Adiponectin and resistin are associated with risk for myelodysplastic syndrome, independently from the insulin-like growth factor-I (IGF-I) system. Eur J Cancer. 2008;44:1744–53. https://doi.org/10.1016/j.ejca.2008.04.015.
Dalamaga M, Karmaniolas K, Matekovits A, Migdalis I, Papadavid E. Cutaneous manifestations in relation to immunologic parameters in a cohort of primary myelodysplastic syndrome patients. J Eur Acad Dermatol Venereol. 2008;22:543–8. https://doi.org/10.1111/j.1468-3083.2007.02520.x.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–84.e6. https://doi.org/10.1016/j.stem.2017.02.009.
Senjo H, Onozawa M, Hidaka D, Yokoyama S, Yamamoto S, Tsutsumi Y, et al. High CRP-albumin ratio predicts poor prognosis in transplant ineligible elderly patients with newly diagnosed acute myeloid leukemia. Sci Rep. 2022;12:8885. https://doi.org/10.1038/s41598-022-12813-1.
Tang HN, Pan BH, Wang L, Zhu HY, Fan L, Xu W, et al. C-reactive protein-to-albumin ratio is an independent poor prognostic factor in newly diagnosed chronic lymphocytic leukaemia: A clinical analysis of 322 cases. Transl Oncol. 2021;14:101035. https://doi.org/10.1016/j.tranon.2021.101035.
Stevens AM, Miller JM, Munoz JO, Gaikwad AS, Redell MS. Interleukin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017;1:1387–97. https://doi.org/10.1182/bloodadvances.2017007856.
Sharma K, Singh U, Rai M, Shukla J, Gupta V, Narayan G, et al. Interleukin 6 and disease transformation in chronic myeloid leukemia: A Northeast Indian population study. J Cancer Res Ther. 2020;16:30–3. https://doi.org/10.4103/jcrt.JCRT_137_17.
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa M, Kumano K, Arai S, et al. Positive feedback between NF-κB and TNF-α promotes leukemia-initiating cell capacity. J Clin Invest. 2014;124:528–42. https://doi.org/10.1172/jci68101.
Verma S, Singh A, Yadav G, Kushwaha R, Ali W, Verma SP, et al. Serum tumor necrosis factor-alpha levels in acute leukemia and its prognostic significance. Cureus. 2022;14:e24835. https://doi.org/10.7759/cureus.24835.
Picó C, Palou M, Pomar CA, Rodríguez AM, Palou A. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022;23:13–30. https://doi.org/10.1007/s11154-021-09687-5.
Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez-Williams G, et al. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: Proliferative and anti-apoptotic activities. Blood. 1999;93:1668–76.
Hamed NA, Sharaki OA, Zeidan MM. Leptin in acute leukaemias: Relationship to interleukin-6 and vascular endothelial growth factor. Egypt J Immunol. 2003;10:57–66.
Yilmaz M, Kis C, Ceylan NO, Okan V, Pehlivan M, Kuçukosmanoglu E, et al. Serum leptin level in acute myeloid leukemia patients. Hematology. 2008;13:21–3. https://doi.org/10.1179/102453308x315771.
Pamuk GE, Demir M, Harmandar F, Yesil Y, Turgut B, Vural O. Leptin and resistin levels in serum of patients with hematologic malignancies: Correlation with clinical characteristics. Exp Oncol. 2006;28:241–4.
Bansal P, Ghalaut VS, Sharma TK, Ghalaut PS, Dokwal S, Ghalaut R, et al. Status of leptin in MBCR-ABL p210 positive chronic myeloid leukemia patients before and after imatinib therapy: A conflicting scenario. Clin Lab. 2014;60:1845–52. https://doi.org/10.7754/clin.lab.2014.140126.
Forny-Germano L, De Felice FG, Vieira M. The role of leptin and adiponectin in obesity-associated cognitive decline and alzheimer's disease. Front Neurosci. 2018;12:1027. https://doi.org/10.3389/fnins.2018.01027.
Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. https://doi.org/10.1155/2014/658913.
Dalamaga M, Nikolaidou A, Karmaniolas K, Hsi A, Chamberland J, Dionyssiou-Asteriou A, et al. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: A case-control study. Oncology. 2007;73:26–32. https://doi.org/10.1159/000120995.
Karampela I, Christodoulatos GS, Kandri E, Antonakos G, Vogiatzakis E, Dimopoulos G, et al. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: A prospective observational study. Cytokine. 2019;119:62–70. https://doi.org/10.1016/j.cyto.2019.03.002.
Huang X, Yang Z. Resistin's, obesity and insulin resistance: The continuing disconnect between rodents and humans. J Endocrinol Invest. 2016;39:607–15. https://doi.org/10.1007/s40618-015-0408-2.
Marouga A, Dalamaga M, Kastania AN, Antonakos G, Thrasyvoulides A, Kontelia G, et al. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab. 2013;59:1121–8. https://doi.org/10.7754/clin.lab.2012.121112.
Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022;10:10840–51. https://doi.org/10.12998/wjcc.v10.i30.10840.
Budakoti M, Panwar AS, Molpa D, Singh RK, Büsselberg D, Mishra AP, et al. Micro-RNA: The darkhorse of cancer. Cell Signal. 2021;83:109995. https://doi.org/10.1016/j.cellsig.2021.109995.
Gharanei S, Shabir K, Brown JE, Weickert MO, Barber TM, Kyrou I, et al. Regulatory microRNAs in brown, brite and white adipose tissue. Cells. 2020:9. https://doi.org/10.3390/cells9112489.
Peng B, Theng PY, Le MTN. Essential functions of miR-125b in cancer. Cell Prolif. 2021;54:e12913. https://doi.org/10.1111/cpr.12913.
Al Azzouny MA, Behiry EG, Behairy OG, Abd Ellraouf HA, Elfallah AA. Serum microRNA-486-5p expression in obese Egyptian children and its possible association with fatty liver. Diabetes Metab Syndr. 2021;15:102258. https://doi.org/10.1016/j.dsx.2021.102258.
Wang LS, Li L, Li L, Chu S, Shiang KD, Li M, et al. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood. 2015;125:1302–13. https://doi.org/10.1182/blood-2014-06-581926.
Meerson A, Traurig M, Ossowski V, Fleming JM, Mullins M, Baier LJ. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia. 2013;56:1971–9. https://doi.org/10.1007/s00125-013-2950-9.
Chan GCK, Than WH, Kwan BCH, Lai KB, Chan RCK, Teoh JYC, et al. Adipose and plasma microRNAs miR-221 and 222 associate with obesity, insulin resistance, and new onset diabetes after peritoneal dialysis. Nutrients. 2022:14. https://doi.org/10.3390/nu14224889.
Kotani A, Ha D, Schotte D, den Boer ML, Armstrong SA, Lodish HF. A novel mutation in the miR-128b gene reduces miRNA processing and leads to glucocorticoid resistance of MLL-AF4 acute lymphocytic leukemia cells. Cell Cycle. 2010;9:1037–42. https://doi.org/10.4161/cc.9.6.11011.
Jiang X, Cheng Y, Hu C, Zhang A, Ren Y, Xu X. MicroRNA-221 sensitizes chronic myeloid leukemia cells to imatinib by targeting STAT5. Leuk Lymphoma. 2019;60:1709–20. https://doi.org/10.1080/10428194.2018.1543875.
Ferracin M, Zagatti B, Rizzotto L, Cavazzini F, Veronese A, Ciccone M, et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol Cancer. 2010;9:123. https://doi.org/10.1186/1476-4598-9-123.
Al-Rawaf HA. Circulating microRNAs and adipokines as markers of metabolic syndrome in adolescents with obesity. Clin Nutr. 2019;38:2231–8. https://doi.org/10.1016/j.clnu.2018.09.024.
Zhang Y, Liu Y, Xu X. Upregulation of miR-142-3p improves drug sensitivity of acute myelogenous leukemia through reducing p-glycoprotein and repressing autophagy by targeting HMGB1. Transl Oncol. 2017;10:410–8. https://doi.org/10.1016/j.tranon.2017.03.003.
Pepe MG, Ginzton NH, Lee PD, Hintz RL, Greenberg PL. Receptor binding and mitogenic effects of insulin and insulinlike growth factors I and II for human myeloid leukemic cells. J Cell Physiol. 1987;133:219–27. https://doi.org/10.1002/jcp.1041330204.
Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: Rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–82. https://doi.org/10.1182/blood-2007-03-080796.
Rodrigues Alves APN, Fernandes JC, Fenerich BA, Coelho-Silva JL, Scheucher PS, Simões BP, et al. IGF1R/IRS1 targeting has cytotoxic activity and inhibits PI3K/AKT/mTOR and MAPK signaling in acute lymphoblastic leukemia cells. Cancer Lett. 2019;456:59–68. https://doi.org/10.1016/j.canlet.2019.04.030.
Artico LL, Laranjeira ABA, Campos LW, Corrêa JR, Zenatti PP, Carvalheira JBC, et al. Physiologic IGFBP7 levels prolong IGF1R activation in acute lymphoblastic leukemia. Blood Adv. 2021;5:3633–46. https://doi.org/10.1182/bloodadvances.2020003627.
Lakshmikuttyamma A, Pastural E, Takahashi N, Sawada K, Sheridan DP, DeCoteau JF, et al. Bcr-Abl induces autocrine IGF-1 signaling. Oncogene. 2008;27:3831–44. https://doi.org/10.1038/onc.2008.8.
Nepstad I, Hatfield KJ, Grønningsæter IS, Reikvam H. The PI3K-Akt-mTOR signaling pathway in human acute myeloid leukemia (AML) cells. Int J Mol Sci. 2020:21. https://doi.org/10.3390/ijms21082907.
Neri LM, Cani A, Martelli AM, Simioni C, Junghanss C, Tabellini G, et al. Targeting the PI3K/Akt/mTOR signaling pathway in B-precursor acute lymphoblastic leukemia and its therapeutic potential. Leukemia. 2014;28:739–48. https://doi.org/10.1038/leu.2013.226.
Adam E, Kim HN, Gang EJ, Schnair C, Lee S, Lee S, et al. The PI3Kδ inhibitor idelalisib inhibits homing in an in vitro and in vivo model of B ALL. Cancers (Basel). 2017:9. https://doi.org/10.3390/cancers9090121.
Place AE, Pikman Y, Stevenson KE, Harris MH, Pauly M, Sulis ML, et al. Phase I trial of the mTOR inhibitor everolimus in combination with multi-agent chemotherapy in relapsed childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e27062. https://doi.org/10.1002/pbc.27062.
Tan P, Tiong IS, Fleming S, Pomilio G, Cummings N, Droogleever M, et al. The mTOR inhibitor everolimus in combination with azacitidine in patients with relapsed/refractory acute myeloid leukemia: A phase Ib/II study. Oncotarget. 2017;8:52269–80. https://doi.org/10.18632/oncotarget.13699.
Kuwatsuka Y, Minami M, Minami Y, Sugimoto K, Hayakawa F, Miyata Y, et al. The mTOR inhibitor, everolimus (RAD001), overcomes resistance to imatinib in quiescent Ph-positive acute lymphoblastic leukemia cells. Blood Cancer J. 2011;1:e17. https://doi.org/10.1038/bcj.2011.16.
Silic-Benussi M, Sharova E, Ciccarese F, Cavallari I, Raimondi V, Urso L, et al. mTOR inhibition downregulates glucose-6-phosphate dehydrogenase and induces ROS-dependent death in T-cell acute lymphoblastic leukemia cells. Redox Biol. 2022;51:102268. https://doi.org/10.1016/j.redox.2022.102268.
Tasian SK, Silverman LB, Whitlock JA, Sposto R, Loftus JP, Schafer ES, et al. Temsirolimus combined with cyclophosphamide and etoposide for pediatric patients with relapsed/refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium trial (TACL 2014-001). Haematologica. 2022;107:2295–303. https://doi.org/10.3324/haematol.2021.279520.
Rheingold SR, Tasian SK, Whitlock JA, Teachey DT, Borowitz MJ, Liu X, et al. A phase 1 trial of temsirolimus and intensive re-induction chemotherapy for 2nd or greater relapse of acute lymphoblastic leukaemia: A Children's Oncology Group study (ADVL1114). Br J Haematol. 2017;177:467–74. https://doi.org/10.1111/bjh.14569.
Scotland S, Micklow E, Wang Z, Boutzen H, Récher C, Danet-Desnoyers G, et al. Metformin for therapeutic intervention in acute myeloid leukemia. Blood. 2010;116:4351. https://doi.org/10.1182/blood.V116.21.4351.4351.
Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116:4262–73. https://doi.org/10.1182/blood-2010-02-269837.
Rosilio C, Lounnas N, Nebout M, Imbert V, Hagenbeek T, Spits H, et al. The metabolic perturbators metformin, phenformin and AICAR interfere with the growth and survival of murine PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells. Cancer Lett. 2013;336:114–26. https://doi.org/10.1016/j.canlet.2013.04.015.
Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–402. https://doi.org/10.1182/blood-2011-01-332783.
Martinez Marignac VL, Smith S, Toban N, Bazile M, Aloyz R. Resistance to Dasatinib in primary chronic lymphocytic leukemia lymphocytes involves AMPK-mediated energetic re-programming. Oncotarget. 2013;4:2550–66. https://doi.org/10.18632/oncotarget.1508.
Bruno S, Ledda B, Tenca C, Ravera S, Orengo AM, Mazzarello AN, et al. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. Oncotarget. 2015;6:22624–40. https://doi.org/10.18632/oncotarget.4168.
Adekola KU, Dalva Aydemir S, Ma S, Zhou Z, Rosen ST, Shanmugam M. Investigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk Lymphoma. 2015;56:450–9. https://doi.org/10.3109/10428194.2014.922180.
Tseng CH. Metformin use and leukemia risk in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2020;11:541090. https://doi.org/10.3389/fendo.2020.541090.
Sugimura A, Kiriyama Y, Nochi H, Tsuchiya H, Tamoto K, Sakurada Y, et al. Troglitazone suppresses cell growth of myeloid leukemia cell lines by induction of p21WAF1/CIP1 cyclin-dependent kinase inhibitor. Biochem Biophys Res Commun. 1999;261:833–7. https://doi.org/10.1006/bbrc.1999.1049.
Hirase N, Yanase T, Mu Y, Muta K, Umemura T, Takayanagi R, et al. Thiazolidinedione induces apoptosis and monocytic differentiation in the promyelocytic leukemia cell line HL60. Oncology. 1999;57(Suppl 2):17–26. https://doi.org/10.1159/000055271.
Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther. 2004;3:1249–62.
Liu JJ, Huang RW, Lin DJ, Peng J, Wu XY, Lin Q, et al. Expression of survivin and bax/bcl-2 in peroxisome proliferator activated receptor-gamma ligands induces apoptosis on human myeloid leukemia cells in vitro. Ann Oncol. 2005;16:455–9. https://doi.org/10.1093/annonc/mdi077.
Takenokuchi M, Saigo K, Nakamachi Y, Kawano S, Hashimoto M, Fujioka T, et al. Troglitazone inhibits cell growth and induces apoptosis of B-cell acute lymphoblastic leukemia cells with t(14;18). Acta Haematol. 2006;116:30–40. https://doi.org/10.1159/000092345.
Saiki M, Hatta Y, Yamazaki T, Itoh T, Enomoto Y, Takeuchi J, et al. Pioglitazone inhibits the growth of human leukemia cell lines and primary leukemia cells while sparing normal hematopoietic stem cells. Int J Oncol. 2006;29:437–43.
Prost S, Relouzat F, Spentchian M, Ouzegdouh Y, Saliba J, Massonnet G, et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525:380–3. https://doi.org/10.1038/nature15248.
Scatena R, Nocca G, Sole PD, Rumi C, Puggioni P, Remiddi F, et al. Bezafibrate as differentiating factor of human myeloid leukemia cells. Cell Death Differ. 1999;6:781–7. https://doi.org/10.1038/sj.cdd.4400551.
Liu H, Zang C, Fenner MH, Liu D, Possinger K, Koeffler HP, et al. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARalpha/gamma ligand TZD18. Blood. 2006;107:3683–92. https://doi.org/10.1182/blood-2005-05-2103.
Friedman DR, Magura LA, Warren HA, Harrison JD, Diehl LF, Weinberg JB. Statin use and need for therapy in chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:2295–8. https://doi.org/10.3109/10428194.2010.520050.
Shanafelt TD, Rabe KG, Kay NE, Zent CS, Call TG, Slager SL, et al. Statin and non-steroidal anti-inflammatory drug use in relation to clinical outcome among patients with Rai stage 0 chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:1233–40. https://doi.org/10.3109/10428194.2010.486877.
Podhorecka M, Halicka D, Klimek P, Kowal M, Chocholska S, Dmoszynska A. Simvastatin and purine analogs have a synergic effect on apoptosis of chronic lymphocytic leukemia cells. Ann Hematol. 2010;89:1115–24. https://doi.org/10.1007/s00277-010-0988-z.
Yavasoglu I, Sargin G, Kadikoylu G, Karul A, Bolaman Z. The activity of atorvastatin and rosiglitazone on CD38, ZAP70 and apoptosis in lymphocytes of B-cell chronic lymphocytic leukemia in vitro. Med Oncol. 2013;30:603. https://doi.org/10.1007/s12032-013-0603-y.
Chae YK, Trinh L, Jain P, Wang X, Rozovski U, Wierda WG, et al. Statin and aspirin use is associated with improved outcome of FCR therapy in relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:1424–6. https://doi.org/10.1182/blood-2013-07-517102.
Chow S, Buckstein R, Spaner DE. A link between hypercholesterolemia and chronic lymphocytic leukemia. Leuk Lymphoma. 2016;57:797–802. https://doi.org/10.3109/10428194.2015.1088651.
Henslee AB, Steele TA. Combination statin and chemotherapy inhibits proliferation and cytotoxicity of an aggressive natural killer cell leukemia. Biomark Res. 2018;6:26. https://doi.org/10.1186/s40364-018-0140-0.
Gimenez N, Tripathi R, Giró A, Rosich L, López-Guerra M, López-Oreja I, et al. Systems biology drug screening identifies statins as enhancers of current therapies in chronic lymphocytic leukemia. Sci Rep. 2020;10:22153. https://doi.org/10.1038/s41598-020-78315-0.
Righolt CH, Zhang G, Ye X, Banerji V, Johnston JB, Gibson S, et al. Statin use and chronic lymphocytic leukemia incidence: A nested case-control study in Manitoba, Canada. Cancer Epidemiol Biomarkers Prev. 2019;28:1495–501. https://doi.org/10.1158/1055-9965.Epi-19-0107.
Jang HJ, Woo YM, Naka K, Park JH, Han HJ, Kim HJ, et al. Statins enhance the molecular response in chronic myeloid leukemia when combined with tyrosine kinase inhibitors. Cancers (Basel). 2021:13. https://doi.org/10.3390/cancers13215543.
Brånvall E, Ekberg S, Eloranta S, Wästerlid T, Birmann BM, Smedby KE. Statin use and survival in 16 098 patients with non-Hodgkin lymphoma or chronic lymphocytic leukaemia treated in the rituximab era. Br J Haematol. 2021;195:552–60. https://doi.org/10.1111/bjh.17733.
Bellosillo B, Piqué M, Barragán M, Castaño E, Villamor N, Colomer D, et al. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406–14.
Weiss JR, Baker JA, Baer MR, Menezes RJ, Nowell S, Moysich KB. Opposing effects of aspirin and acetaminophen use on risk of adult acute leukemia. Leuk Res. 2006;30:164–9. https://doi.org/10.1016/j.leukres.2005.06.023.
Iglesias-Serret D, Piqué M, Barragán M, Cosialls AM, Santidrián AF, González-Gironès DM, et al. Aspirin induces apoptosis in human leukemia cells independently of NF-kappaB and MAPKs through alteration of the Mcl-1/Noxa balance. Apoptosis. 2010;15:219–29. https://doi.org/10.1007/s10495-009-0424-9.
Ross JA, Blair CK, Cerhan JR, Soler JT, Hirsch BA, Roesler MA, et al. Nonsteroidal anti-inflammatory drug and acetaminophen use and risk of adult myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 2011;20:1741–50. https://doi.org/10.1158/1055-9965.Epi-11-0411.
Liang S, Zhou X, Cai D, Rodrigues-Lima F, Chi J, Wang L. Network pharmacology and experimental validation reveal the effects of chidamide combined with aspirin on acute myeloid leukemia-myelodysplastic syndrome cells through PI3K/AKT pathway. Front Cell Dev Biol. 2021;9:685954. https://doi.org/10.3389/fcell.2021.685954.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–85. https://doi.org/10.1007/s00125-017-4342-z.
Chang WH, Lai AG. An integrative pan-cancer investigation reveals common genetic and transcriptional alterations of AMPK pathway genes as important predictors of clinical outcomes across major cancer types. BMC Cancer. 2020;20:773. https://doi.org/10.1186/s12885-020-07286-2.
Fruchart JC, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc). 2006;42:39–64. https://doi.org/10.1358/dot.2006.42.1.963528.
Winiarska M, Bil J, Wilczek E, Wilczynski GM, Lekka M, Engelberts PJ, et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008;5:e64. https://doi.org/10.1371/journal.pmed.0050064.
Tsilingiris D, Nasiri-Ansari N, Spyrou N, Magkos F, Dalamaga M. Management of hematologic malignancies in the era of COVID-19 pandemic: Pathogenetic mechanisms, impact of obesity, perspectives, and challenges. Cancers (Basel). 2022:14. https://doi.org/10.3390/cancers14102494.
Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190:e16–20. https://doi.org/10.1111/bjh.16801.
Paul S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD, et al. Treating leukemia in the time of COVID-19. Acta Haematol. 2021;144:132–45. https://doi.org/10.1159/000508199.
Shen Y, Freeman JA, Holland J, Naidu K, Solterbeck A, Van Bilsen N, et al. Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion. Blood. 2022;140:2709–21. https://doi.org/10.1182/blood.2022017814.
Furlan A, Forner G, Cipriani L, Vian E, Rigoli R, Gherlinzoni F, et al. COVID-19 in B cell-depleted patients after rituximab: A diagnostic and therapeutic challenge. Front Immunol. 2021;12:763412. https://doi.org/10.3389/fimmu.2021.763412.
Thornton CS, Huntley K, Berenger BM, Bristow M, Evans DH, Fonseca K, et al. Prolonged SARS-CoV-2 infection following rituximab treatment: Clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control. 2022;11:28. https://doi.org/10.1186/s13756-022-01067-1.
Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–9. https://doi.org/10.1016/j.ccell.2021.09.001.
•• Larson EA, Dalamaga M, Magkos F. The role of exercise in obesity-related cancers: Current evidence and biological mechanisms. Semin Cancer Biol. 2023;91:16–26. https://doi.org/10.1016/j.semcancer.2023.02.008. This review summarizes recent evidence on the effect of physical activity on obesity-related cancer prevention and survival.
Verde L, Dalamaga M, Capó X, Annunziata G, Hassapidou M, Docimo A, et al. The antioxidant potential of the Mediterranean diet as a predictor of weight loss after a very low-calorie ketogenic diet (VLCKD) in women with overweight and obesity. Antioxidants (Basel). 2022:12. https://doi.org/10.3390/antiox12010018.
Muscogiuri G, Verde L, Sulu C, Katsiki N, Hassapidou M, Frias-Toral E, et al. Mediterranean diet and obesity-related disorders: What is the evidence? Curr Obes Rep. 2022;11:287–304. https://doi.org/10.1007/s13679-022-00481-1.
Rokou A, Eleftheriou A, Tsigalou C, Apessos I, Nena E, Dalamaga M, et al. Effect of the implementation of a structured diet management plan on the severity of obstructive sleep apnea: A systematic review. Curr Nutr Rep. 2023;12:26–38. https://doi.org/10.1007/s13668-022-00445-w.
Lempesis IG, Liu J, Dalamaga M. The catcher in the gut: Tirzepatide, a dual incretin analog for the treatment of type 2 diabetes mellitus and obesity. Metabol Open. 2022;16:100220. https://doi.org/10.1016/j.metop.2022.100220.
Vallianou NG, Tsilingiris D, Kounatidis D, Lempesis IG, Karampela I, Dalamaga M. Sodium-glucose cotransporter-2 inhibitors in obesity and associated cardiometabolic disorders: Where do we stand? Pol. Arch Intern Med. 2022:132. https://doi.org/10.20452/pamw.16342.
Kounatidis D, Vallianou NG, Tsilingiris D, Christodoulatos GS, Geladari E, Stratigou T, et al. Therapeutic potential of GLP-2 analogs in gastrointestinal disorders: Current knowledge, nutritional aspects, and future perspectives. Curr Nutr Rep. 2022;11:618–42. https://doi.org/10.1007/s13668-022-00433-0.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
MD conceived the idea of the review, organized its plan, and supervised the study. DT, NV, NS, DK, and MD wrote parts of the manuscript. DT, IK, NV, NS, and GSC performed literature search. DT, DK, and NV prepared the tables. GSC made the figures. DT, NV, and MD reviewed the manuscript. The corresponding author states that all authors meet authorship criteria.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Institutional Review Board Statement
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsilingiris, D., Vallianou, N.G., Spyrou, N. et al. Obesity and Leukemia: Biological Mechanisms, Perspectives, and Challenges. Curr Obes Rep 13, 1–34 (2024). https://doi.org/10.1007/s13679-023-00542-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00542-z