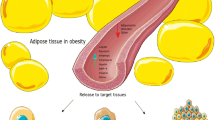
Abstract
Purpose
The current review shows evidence for the role of adipokines in breast cancer (BC) pathogenesis summarizing the mechanisms underlying the association between adipokines and breast malignancy. Special emphasis is given also on intriguing insights into the relationship between obesity and BC as well as on the role of novel adipokines in BC development.
Recent Findings
Recent evidence has underscored the role of the triad of obesity, insulin resistance, and adipokines in postmenopausal BC. Adipokines exert independent and joint effects on activation of major intracellular signal networks implicated in BC cell proliferation, growth, survival, invasion, and metastasis, particularly in the context of obesity, considered a systemic endocrine dysfunction characterized by chronic inflammation. To date, more than 10 adipokines have been linked to BC, and this catalog is continuously increasing. The majority of circulating adipokines, such as leptin, resistin, visfatin, apelin, lipocalin 2, osteopontin, and oncostatin M, is elevated in BC, while some adipokines such as adiponectin and irisin (adipo-myokine) are generally decreased in BC and considered protective against breast carcinogenesis.
Summary
Further evidence from basic and translational research is necessary to delineate the ontological role of adipokines and their interplay in BC pathogenesis. More large-scale clinical and longitudinal studies are awaited to assess their clinical utility in BC prognosis and follow-up. Finally, novel more effective and safer adipokine-centered therapeutic strategies could pave the way for targeted oncotherapy.


Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-coA carboxylase
- ADSCs:
-
Adipose-derived mesenchymal stem cells
- AdipoR1/R2:
-
adiponectin receptor 1/ 2
- Akt: v-:
-
Akt murine thymoma viral oncogene homolog
- AMPK:
-
5′ AMP-activated protein kinase
- APPL1:
-
Adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1
- bax:
-
Bcl-2 associated X protein
- BC:
-
breast cancer
- bcl-xL:
-
B cell lymphoma-extra large
- BMI:
-
Body mass index
- CAP1:
-
Adenylyl cyclase–associated protein 1
- CPT1B:
-
Carnitine palmitoyltransferase 1B
- CSC:
-
Cancer stem cell
- c-Src:
-
Proto-oncogene tyrosine-proteine kinase Src
- DM:
-
Diabetes mellitus
- EMT:
-
Epithelial-mesenchymal transition
- eNampt::
-
Extracellular nicotinamide phosphoribosyl-transferase (eNampt)
- ER:
-
Estrogen receptor
- ERK 1/2:
-
Extracellular signal-regulated kinase 1/2
- FAO:
-
Fatty acid b-oxidation
- FASN:
-
Fatty acid synthase
- GRP78:
-
Glucose-regulated protein 78
- GTP:
-
Guanosine-5′-triphosphate
- HER:
-
Human epidermal growth factor receptor
- HIF-1a:
-
Hypoxia-inducible factor-1a
- HR:
-
Hormone receptor
- HRT:
-
Hormone replacement therapy
- hsCRP:
-
High-sensitive C-reactive protein
- IL:
-
Interleukin
- IARC:
-
International Agency for Research on Cancer
- IGF:
-
Insulin-like growth factor
- IRS:
-
Insulin receptor substrate
- JAK:
-
Janus kinase
- JNK:
-
Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MMTV:
-
Mammary tumor virus
- Lcn2:
-
Lipocalin 2
- LEPR:
-
Leptin receptor
- LIFR:
-
Leukemia inhibitory receptor
- LKB1:
-
else known as STK11 (serine/threonine kinase 11)
- MCF-7:
-
Michigan Cancer Foundation 7
- miR:
-
Micro-RNA
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mammalian target of rapamycin
- MO25:
-
Scaffolding mouse 25 protein
- NAD:
-
Nicotinamide adenine dinucleotide
- Nampt:
-
Nicotinamide phosphoribosyl-transferase
- NF-κB:
-
nuclear factor-κB
- NGAL:
-
Neutrophil gelatinase–associated lipocalin
- NILCO:
-
Notch, IL-1, and leptin
- OPN:
-
Osteopontin
- OSM:
-
Oncostatin M
- OSMR:
-
OSM receptor II
- OR:
-
Odds ratio
- PBEF:
-
pre-B cell colony–enhancing factor
- PI3K:
-
Phosphatidylinositol 3-kinase
- PPAR:
-
Peroxisome proliferator–activated receptors
- PR:
-
Progesterone receptor
- RBP-4:
-
Retinol-binding protein
- ROCK:
-
Rho-associated coiled coil-containing protein kinase
- SHBG:
-
Sex hormone–binding globulin
- SIRT1:
-
Sirtuin 1
- SMD:
-
Standardized mean difference
- STAT:
-
Signal transducer and activator of transcription
- STRA6:
-
Stimulated by retinoic acid 6
- STRAD:
-
STE20-related adaptor protein
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cellular adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wingless-related integration site
- WC:
-
Waist circumference
- WHR:
-
Waist-to hip ratio
- WCRF/AICR:
-
World Cancer Research Fund/American Institute for Cancer Research
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The state of cancer care in America. 2014: a report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10:119–42. https://doi.org/10.1200/jop.2014.001386.
Pischon T, Nimptsch K. Obesity and risk of cancer: an introductory overview. Recent Results Cancer Res. 2016;208:1–15. https://doi.org/10.1007/978-3-319-42542-9_1.
Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112. https://doi.org/10.3322/caac.21499.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7. https://doi.org/10.1038/ijo.2008.102.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. https://doi.org/10.1056/NEJMsr1606602.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. https://doi.org/10.3322/caac.21349.
Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15. https://doi.org/10.1016/s2213-8587(18)30150-5.
•• Spyrou N, Avgerinos KI, Mantzoros CS, Dalamaga M. Classic and novel adipocytokines at the intersection of obesity and cancer: diagnostic and therapeutic strategies. Curr Obes Rep. 2018;7:260–75. https://doi.org/10.1007/s13679-018-0318-7This review summarizes the association of classic and novel adipokines with cancer giving special emphasis on mechanisms of action and clinical studies.
•• Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001This review shows evidence for the association between obesity and cancer underscoring the role of emerging biological mechanisms.
Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. 2015;22:R365–86. https://doi.org/10.1530/erc-15-0400.
Canchola AJ, Anton-Culver H, Bernstein L, Clarke CA, Henderson K, Ma H, et al. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012. https://doi.org/10.1007/s10552-012-9897-x.
•• Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–97. https://doi.org/10.3322/caac.21405This review summarizes the relationships between obesity and breast cancer development and addresses implicated molecular mechanistic insights and strategies for intervention.
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. https://doi.org/10.1093/epirev/mxt010.
Rosner B, Eliassen AH, Toriola AT, Chen WY, Hankinson SE, Willett WC, et al. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer. 2017;140:2003–14. https://doi.org/10.1002/ijc.30627.
Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.5327.
Namazi N, Irandoost P, Heshmati J, Larijani B, Azadbakht L. The association between fat mass and the risk of breast cancer: a systematic review and meta-analysis. Clin Nutr. 2019;38:1496–503. https://doi.org/10.1016/j.clnu.2018.09.013.
Fagherazzi G, Fabre A, Boutron-Ruault MC, Clavel-Chapelon F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: results from the prospective E3N cohort. Eur J Cancer Prev. 2010;19:120–5. https://doi.org/10.1097/CEJ.0b013e3283354918.
Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587–97. https://doi.org/10.1007/s10549-011-1616-x.
Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. https://doi.org/10.1007/s10549-007-9632-6.
Ford NA, Nunez NP, Holcomb VB, Hursting SD. IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression, epithelial-to-mesenchymal transition, and chemokine expression. Endocr Relat Cancer. 2013;20:39–51. https://doi.org/10.1530/erc-12-0329.
Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res (Phila). 2012;5:930–42. https://doi.org/10.1158/1940-6207.capr-12-0034.
Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–14. https://doi.org/10.1093/annonc/mdu042.
Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203–16. https://doi.org/10.1200/jco.2016.68.4480.
•• Gui Y, Pan Q, Chen X, Xu S, Luo X, Chen L. The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget. 2017;8:75389–99. https://doi.org/10.18632/oncotarget.17853This meta-analysis examines the association of the serum levels of several adipokines and the risk of breast cancer.
Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. https://doi.org/10.1001/jamaoncol.2018.0137.
Bradshaw PT, Cespedes Feliciano EM, Prado CM, Alexeeff S, Albers KB, Chen WY, et al. Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity (Silver Spring). 2019;27:997–1004. https://doi.org/10.1002/oby.22458.
Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: new biomarkers and attractive therapeutic targets. World J Exp Med. 2013;3:34–42. https://doi.org/10.5493/wjem.v3.i3.34.
Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–9. https://doi.org/10.1016/j.tem.2011.10.003.
Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. https://doi.org/10.1210/er.2011-1015.
Kassi E, Dalamaga M, Hroussalas G, Kazanis K, Merantzi G, Zachari A, et al. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas. 2010;67:72–7. https://doi.org/10.1016/j.maturitas.2010.05.004.
Christodoulatos GS, Dalamaga M. Micro-RNAs as clinical biomarkers and therapeutic targets in breast cancer: Quo vadis? World J Clin Oncol. 2014;5:71–81. https://doi.org/10.5306/wjco.v5.i2.71.
Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause. 2013;20:845–51. https://doi.org/10.1097/GME.0b013e31827f06dc.
Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause. 2011;18:1198–204. https://doi.org/10.1097/gme.0b013e31821e21f5.
Dalamaga M, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46:584–90. https://doi.org/10.1016/j.clinbiochem.2013.01.001.
Dalamaga M. Nicotinamide phosphoribosyl-transferase/visfatin: a missing link between overweight/obesity and postmenopausal breast cancer? Potential preventive and therapeutic perspectives and challenges. Med Hypotheses. 2012;79:617–21. https://doi.org/10.1016/j.mehy.2012.07.036.
Dalamaga M, Archondakis S, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, et al. Could serum visfatin be a potential biomarker for postmenopausal breast cancer? Maturitas. 2012;71:301–8. https://doi.org/10.1016/j.maturitas.2011.12.013.
Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–66. https://doi.org/10.1016/j.tcb.2012.08.001.
Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017;19:8. https://doi.org/10.1186/s13058-016-0791-4.
Samuel SM, Varghese E, Varghese S, Busselberg D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat Rev. 2018;70:98–111. https://doi.org/10.1016/j.ctrv.2018.08.004.
Cha YJ, Koo JS. Adipokines as therapeutic targets in breast cancer treatment. Expert Opin Ther Targets. 2018;22:941–53. https://doi.org/10.1080/14728222.2018.1538356.
Dalamaga M, Christodoulatos GS, Mantzoros CS. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. https://doi.org/10.1016/j.metabol.2018.01.001.
Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. https://doi.org/10.1210/jc.2003-031804.
Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. https://doi.org/10.1210/er.2012-1053.
Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. https://doi.org/10.1016/j.cmet.2013.05.010.
Lee CH, Woo YC, Wang Y, Yeung CY, Xu A, Lam KS. Obesity, adipokines and cancer: an update. Clin Endocrinol. 2015;83:147–56. https://doi.org/10.1111/cen.12667.
Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. https://doi.org/10.1016/j.febslet.2007.11.070.
Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. https://doi.org/10.1074/jbc.M501149200.
Ando S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, et al. Obesity, Leptin and breast cancer: epidemiological evidence and proposed mechanisms. Cancers (Basel). 2019;11. https://doi.org/10.3390/cancers11010062.
Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–14. https://doi.org/10.1093/emboj/cdg490.
Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. https://doi.org/10.1146/annurev.biochem.75.103004.142702.
Panno ML, Naimo GD, Spina E, Ando S, Mauro L. Different molecular signaling sustaining adiponectin action in breast cancer. Curr Opin Pharmacol. 2016;31:1–7. https://doi.org/10.1016/j.coph.2016.08.001.
Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, et al. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–9.
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–70. https://doi.org/10.1158/0008-5472.can-06-1969.
Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–9. https://doi.org/10.1038/sj.bjc.6604166.
Nakayama S, Miyoshi Y, Ishihara H, Noguchi S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res Treat. 2008;112:405–10. https://doi.org/10.1007/s10549-007-9874-3.
Mauro L, Pellegrino M, De Amicis F, Ricchio E, Giordano F, Rizza P, et al. Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle. 2014;13:553–64. https://doi.org/10.4161/cc.27455.
Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, et al. Estrogen receptor-alpha drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015;29:2150–60. https://doi.org/10.1096/fj.14-262808.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. https://doi.org/10.1016/j.ccr.2012.02.014.
Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–70. https://doi.org/10.2217/fon.09.174.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. https://doi.org/10.1038/ncb2329.
Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. https://doi.org/10.1038/nrc2676.
Mauro L, Naimo GD, Gelsomino L, Malivindi R, Bruno L, Pellegrino M, et al. Uncoupling effects of estrogen receptor alpha on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. 2018;32:4343–55. https://doi.org/10.1096/fj.201701315R.
Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49:3–13. https://doi.org/10.1111/cpr.12233.
Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150:685–95. https://doi.org/10.1007/s10549-015-3326-2.
Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383:13–20. https://doi.org/10.1007/s11010-013-1746-z.
• Gu L, Cao C, Fu J, Li Q, Li DH, Chen MY. Serum adiponectin in breast cancer: a meta-analysis. Medicine (Baltimore). 2018;97:e11433. https://doi.org/10.1097/md.0000000000011433This very recent meta-analysis demonstrates the association between low adiponectin levels and breast cancer in both premenopausal and postmenopausal women.
Liu LY, Wang M, Ma ZB, Yu LX, Zhang Q, Gao DZ, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One. 2013;8:e73183. https://doi.org/10.1371/journal.pone.0073183.
Ye J, Jia J, Dong S, Zhang C, Yu S, Li L, et al. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev. 2014;23:158–65. https://doi.org/10.1097/CEJ.0b013e328364f293.
Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:1226–36. https://doi.org/10.1093/ije/dyu088.
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–84. https://doi.org/10.1152/ajpendo.00315.2011.
Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64:1–4. https://doi.org/10.1016/j.metabol.2014.10.023.
Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Invest. 2015;21:57–74. https://doi.org/10.1515/hmbci-2014-0037.
Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. https://doi.org/10.1677/erc.1.01169.
Choi JH, Park SH, Leung PC, Choi KC. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab. 2005;90:207–10. https://doi.org/10.1210/jc.2004-0297.
Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene. 2000;19:1379–85. https://doi.org/10.1038/sj.onc.1203443.
Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–93.
Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila). 2011;4:1449–56. https://doi.org/10.1158/1940-6207.capr-11-0125.
Garcia-Robles MJ, Segura-Ortega JE, Fafutis-Morris M. The biology of leptin and its implications in breast cancer: a general view. J Interf Cytokine Res. 2013;33:717–27. https://doi.org/10.1089/jir.2012.0168.
Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. https://doi.org/10.1016/j.ejca.2010.09.005.
Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–76. https://doi.org/10.1074/jbc.M301695200.
Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–15. https://doi.org/10.1074/jbc.M313191200.
Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. https://doi.org/10.1016/j.eururo.2012.11.013.
Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, Morales S, et al. Evidences that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 2007;67:3412–21. https://doi.org/10.1158/0008-5472.can-06-2890.
Raut PK, Choi DY, Kim SH, Hong JT, Kwon TK, Jeong JH, et al. Estrogen receptor signaling mediates leptin-induced growth of breast cancer cells via autophagy induction. Oncotarget. 2017;8:109417–35. https://doi.org/10.18632/oncotarget.22684.
Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am J Pathol. 2010;177:3133–44. https://doi.org/10.2353/ajpath.2010.100595.
Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. https://doi.org/10.1530/erc-11-0102.
Chang CC, Wu MJ, Yang JY, Camarillo IG, Chang CJ. Leptin-STAT3-G9a signaling promotes obesity-mediated breast cancer progression. Cancer Res. 2015;75:2375–86. https://doi.org/10.1158/0008-5472.can-14-3076.
Giordano C, Vizza D, Panza S, Barone I, Bonofiglio D, Lanzino M, et al. Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol Oncol. 2013;7:379–91. https://doi.org/10.1016/j.molonc.2012.11.002.
Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. https://doi.org/10.1371/journal.pone.0021467.
Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6:43–55. https://doi.org/10.5662/wjm.v6.i1.43.
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:1357. https://doi.org/10.1016/j.cmet.2018.04.018.
• Pan H, Deng LL, Cui JQ, Shi L, Yang YC, Luo JH, et al. Association between serum leptin levels and breast cancer risk: an updated systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11345. https://doi.org/10.1097/md.0000000000011345This recent meta-analysis has shown that higher leptin levels are associated with increased risk for breast cancer, especially for overweight/obese and postmenopausal women.
Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta-analysis. PLoS One. 2013;8:e67349. https://doi.org/10.1371/journal.pone.0067349.
Dalamaga M. Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomark Med. 2014;8:107–18. https://doi.org/10.2217/bmm.13.99.
Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–32. https://doi.org/10.1111/j.1476-5381.2011.01369.x.
Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37:589–600. https://doi.org/10.1038/onc.2017.357.
Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, et al. Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms. Cytokine. 2019;120:155–64. https://doi.org/10.1016/j.cyto.2019.04.016.
Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, et al. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep. 2016;6:18923. https://doi.org/10.1038/srep18923.
Liu Z, Shi A, Song D, Han B, Zhang Z, Ma L, et al. Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction. Am J Cancer Res. 2017;7:574–83.
Deshmukh SK, Srivastava SK, Zubair H, Bhardwaj A, Tyagi N, Al-Ghadhban A, et al. Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett. 2017;396:21–9. https://doi.org/10.1016/j.canlet.2017.03.010.
Georgiou GP, Provatopoulou X, Kalogera E, Siasos G, Menenakos E, Zografos GC, et al. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast. 2016;29:163–9. https://doi.org/10.1016/j.breast.2016.07.025.
Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol. 2012;125:742–50. https://doi.org/10.1016/j.ygyno.2012.02.032.
Gong WJ, Zheng W, Xiao L, Tan LM, Song J, Li XP, et al. Circulating resistin levels and obesity-related cancer risk: a meta-analysis. Oncotarget. 2016;7:57694–704. https://doi.org/10.18632/oncotarget.11034.
Zhang LQ, Heruth DP, Ye SQ. Nicotinamide Phosphoribosyltransferase in human diseases. J Bioanal Biomed. 2011;3:13–25. https://doi.org/10.4172/1948-593x.1000038.
Duarte-Pereira S, Silva SS, Azevedo L, Castro L, Amorim A, Silva RM. NAMPT and NAPRT1: novel polymorphisms and distribution of variants between normal tissues and tumor samples. Sci Rep. 2014;4:6311. https://doi.org/10.1038/srep06311.
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. https://doi.org/10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L.
Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. https://doi.org/10.1158/0008-5472.can-09-2465.
Yu-Duan T, Chao-Ping W, Chih-Yu C, Li-Wen L, Tsun-Mei L, Chia-Chang H, et al. Elevated plasma level of visfatin/pre-b cell colony-enhancing factor in male oral squamous cell carcinoma patients. Med Oral Patol Oral Cir Bucal. 2013;18:e180–6. https://doi.org/10.4317/medoral.18574.
Park HJ, Kim SR, Kim SS, Wee HJ, Bae MK, Ryu MH, et al. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget. 2014;5:5087–99. https://doi.org/10.18632/oncotarget.2086.
Gabow PA. Autosomal dominant polycystic kidney disease--more than a renal disease. Am J Kidney Dis. 1990;16:403–13.
Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–23. https://doi.org/10.1182/blood-2014-07-589069.
Behrouzfar K, Alaee M, Nourbakhsh M, Gholinejad Z, Golestani A. Extracellular NAMPT/visfatin causes p53 deacetylation via NAD production and SIRT1 activation in breast cancer cells. Cell Biochem Funct. 2017;35:327–33. https://doi.org/10.1002/cbf.3279.
Gholinejad Z, Kheiripour N, Nourbakhsh M, Ilbeigi D, Behroozfar K, Hesari Z, et al. Extracellular NAMPT/Visfatin induces proliferation through ERK1/2 and AKT and inhibits apoptosis in breast cancer cells. Peptides. 2017;92:9–15. https://doi.org/10.1016/j.peptides.2017.04.007.
Lee YC, Yang YH, Su JH, Chang HL, Hou MF, Yuan SS. High visfatin expression in breast cancer tissue is associated with poor survival. Cancer Epidemiol Biomark Prev. 2011;20:1892–901. https://doi.org/10.1158/1055-9965.epi-11-0399.
Hung AC, Lo S, Hou MF, Lee YC, Tsai CH, Chen YY, et al. Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin Cancer Res. 2016;22:4478–90. https://doi.org/10.1158/1078-0432.ccr-15-2704.
Moi SH, Lee YC, Chuang LY, Yuan SF, Ou-Yang F, Hou MF, et al. Cumulative receiver operating characteristics for analyzing interaction between tissue visfatin and clinicopathologic factors in breast cancer progression. Cancer Cell Int. 2018;18:19. https://doi.org/10.1186/s12935-018-0517-z.
Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: a systematic review and meta-analysis. Obes Res Clin Pract. 2019. https://doi.org/10.1016/j.orcp.2019.03.006.
Carpene C, Dray C, Attane C, Valet P, Portillo MP, Churruca I, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73.
Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9. https://doi.org/10.1007/s12020-011-9507-9.
Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. https://doi.org/10.1210/en.2004-1427.
Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget. 2014;5:4426–37. https://doi.org/10.18632/oncotarget.2032.
Peng X, Li F, Wang P, Jia S, Sun L, Huo H. Apelin-13 induces MCF-7 cell proliferation and invasion via phosphorylation of ERK1/2. Int J Mol Med. 2015;36:733–8. https://doi.org/10.3892/ijmm.2015.2265.
Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 2007;26:7692–9. https://doi.org/10.1038/sj.onc.1210573.
Hu D, Zhu WF, Shen WC, Xia Y, Wu XF, Zhang HJ, et al. Expression of Apelin and Snail protein in breast cancer and their prognostic significance. Zhonghua Bing Li Xue Za Zhi. 2018;47:743–6. https://doi.org/10.3760/cma.j.issn.0529-5807.2018.10.002.
Wang Z, Greeley GH Jr, Qiu S. Immunohistochemical localization of apelin in human normal breast and breast carcinoma. J Mol Histol. 2008;39:121–4. https://doi.org/10.1007/s10735-007-9135-0.
Salman T, Demir L, Varol U, Akyol M, Oflazoglu U, Yildiz Y, et al. Serum apelin levels and body composition changes in breast cancer patients treated with an aromatase inhibitor. J buon. 2016;21:1419–24.
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–88. https://doi.org/10.1074/jbc.M700793200.
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. https://doi.org/10.1210/en.2007-0175.
Shin WJ, Zabel BA, Pachynski RK. Mechanisms and functions of chemerin in cancer: potential roles in therapeutic intervention. Front Immunol. 2018;9:2772. https://doi.org/10.3389/fimmu.2018.02772.
Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev. 2016;17:361–76. https://doi.org/10.1111/obr.12377.
El-Sagheer G, Gayyed M, Ahmad A, Abd El-Fattah A, Mohamed M. Expression of chemerin correlates with a poor prognosis in female breast cancer patients. Breast Cancer (Dove Med Press). 2018;10:169–76. https://doi.org/10.2147/bctt.s178181.
• Pachynski RK, Wang P, Salazar N, Zheng Y, Nease L, Rosalez J, et al. Chemerin suppresses breast cancer growth by recruiting immune effector cells into the tumor microenvironment. Front Immunol. 2019;10:983. https://doi.org/10.3389/fimmu.2019.00983This study has shown for the first time that increased chemerin expression into the breast cancer milieu can suppress tumor growth by recruiting NK and T cells, thus supporting this approach as a promising immunotherapeutic strategy.
Akin S. Serum chemerin level in breast cancer. Int J Hematol Oncol. 2017;27:127–32. https://doi.org/10.4999/uhod.171847.
Hofmann T, Elbelt U, Stengel A. Irisin as a muscle-derived hormone stimulating thermogenesis--a critical update. Peptides. 2014;54:89–100. https://doi.org/10.1016/j.peptides.2014.01.016.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. https://doi.org/10.1038/nature10777.
Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–37. https://doi.org/10.1038/nrendo.2016.221.
Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–6. https://doi.org/10.1016/j.peptides.2014.09.014.
Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. https://doi.org/10.1371/journal.pone.0060563.
Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136:E197–202. https://doi.org/10.1002/ijc.29142.
Provatopoulou X, Georgiou GP, Kalogera E, Kalles V, Matiatou MA, Papapanagiotou I, et al. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer. 2015;15:898. https://doi.org/10.1186/s12885-015-1898-1.
Zhang ZP, Zhang XF, Li H, Liu TJ, Zhao QP, Huang LH, et al. Serum irisin associates with breast cancer to spinal metastasis. Medicine (Baltimore). 2018;97:e0524. https://doi.org/10.1097/md.0000000000010524.
Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. https://doi.org/10.1042/bj3180001.
•• Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11:8099–106. https://doi.org/10.2147/ott.s181223This review summarizes evidence on the abnormal expression of lipocalin 2 in breast cancer progression, highlights the latest developments of potential lipocalin 2-targeting agents and proposed lipocalin 2-associated molecular mechanisms implicated in breast cancer invasion and metastasis.
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–8. https://doi.org/10.1073/pnas.0810617106.
Oren B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol. 2016;239:274–85. https://doi.org/10.1002/path.4724.
Leng X, Wu Y, Arlinghaus RB. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J Cell Physiol. 2011;226:309–14. https://doi.org/10.1002/jcp.22403.
Yang J, McNeish B, Butterfield C, Moses MA. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013;27:45–50. https://doi.org/10.1096/fj.12-211730.
Wang Y, Zeng T. Neutrophil gelatinase-associated lipocalin protein as a biomarker in the diagnosis of breast cancer: a meta-analysis. Biomed Rep. 2013;1:479–83. https://doi.org/10.3892/br.2013.89.
Sung H, Choi JY, Lee SA, Lee KM, Han S, Jeon S, et al. The association between the preoperative serum levels of lipocalin-2 and matrix metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC Cancer. 2012;12:193. https://doi.org/10.1186/1471-2407-12-193.
Wenners AS, Mehta K, Loibl S, Park H, Mueller B, Arnold N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) predicts response to neoadjuvant chemotherapy and clinical outcome in primary human breast cancer. PLoS One. 2012;7:e45826. https://doi.org/10.1371/journal.pone.0045826.
Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108:389–97. https://doi.org/10.1007/s10549-007-9619-3.
Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessas I, Goussetis E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. https://doi.org/10.1186/1471-2407-9-390.
Cheng G, Sun X, Wang J, Xiao G, Wang X, Fan X, et al. HIC1 silencing in triple-negative breast cancer drives progression through misregulation of LCN2. Cancer Res. 2014;74:862–72. https://doi.org/10.1158/0008-5472.can-13-2420.
Tanaka M, Miyajima A, Oncostatin M. A multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003;149:39–52. https://doi.org/10.1007/s10254-003-0013-1.
Sanchez-Infantes D, White UA, Elks CM, Morrison RF, Gimble JM, Considine RV, et al. Oncostatin m is produced in adipose tissue and is regulated in conditions of obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E217–25. https://doi.org/10.1210/jc.2013-3555.
Richards CD. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013;2013:512103. https://doi.org/10.1155/2013/512103.
Gomez-Lechon MJ. Oncostatin M: signal transduction and biological activity. Life Sci. 1999;65:2019–30.
Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, et al. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res. 2014;74:6806–19. https://doi.org/10.1158/0008-5472.can-14-0160.
West NR, Murray JI, Watson PH. Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer. Oncogene. 2014;33:1485–94. https://doi.org/10.1038/onc.2013.105.
Tawara K, Bolin C, Koncinsky J, Kadaba S, Covert H, Sutherland C, et al. OSM potentiates preintravasation events, increases CTC counts, and promotes breast cancer metastasis to the lung. Breast Cancer Res. 2018;20:53. https://doi.org/10.1186/s13058-018-0971-5.
Tawara K, Scott H, Emathinger J, Wolf C, LaJoie D, Hedeen D, et al. HIGH expression of OSM and IL-6 are associated with decreased breast cancer survival: synergistic induction of IL-6 secretion by OSM and IL-1beta. Oncotarget. 2019;10:2068–85. https://doi.org/10.18632/oncotarget.26699.
West NR, Murphy LC, Watson PH. Oncostatin M suppresses oestrogen receptor-alpha expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer. 2012;19:181–95. https://doi.org/10.1530/erc-11-0326.
Doherty MR, Parvani JG, Tamagno I, Junk DJ, Bryson BL, Cheon HJ, et al. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. 2019;21:54. https://doi.org/10.1186/s13058-019-1136-x.
Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303.
Kahles F, Findeisen HM, Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–93. https://doi.org/10.1016/j.molmet.2014.03.004.
Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomark Prev. 2007;16:1087–97. https://doi.org/10.1158/1055-9965.epi-06-1008.
Zhao H, Chen Q, Alam A, Cui J, Suen KC, Soo AP, et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. https://doi.org/10.1038/s41419-018-0391-6.
Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J Cell Biochem. 2000;78:465–75.
Noti JD. Adherence to osteopontin via alphavbeta3 suppresses phorbol ester-mediated apoptosis in MCF-7 breast cancer cells that overexpress protein kinase C-alpha. Int J Oncol. 2000;17:1237–43. https://doi.org/10.3892/ijo.17.6.1237.
Singhal H, Bautista DS, Tonkin KS, O’Malley FP, Tuck AB, Chambers AF, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–11.
Rudland PS, Platt-Higgins A, El-Tanani M, De Silva RS, Barraclough R, Winstanley JH, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–27.
Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–23.
Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia. 2001;6:419–29.
Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621–32.
Xu K, Tian X, Oh SY, Movassaghi M, Naber SP, Kuperwasser C, et al. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res. 2016;18:14. https://doi.org/10.1186/s13058-016-0674-8.
Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–73. https://doi.org/10.1158/0008-5472.can-14-1990.
• Han B, Huang J, Han Y, Hao J, Wu X, Song H, et al. The microRNA miR-181c enhances chemosensitivity and reduces chemoresistance in breast cancer cells via down-regulating osteopontin. Int J Biol Macromol. 2019;125:544–56. https://doi.org/10.1016/j.ijbiomac.2018.12.075This study provides new insights in developing effective interventions for breast cancer patients with acquired resistance to chemotherapy suggesting that the miR-181c/osteopontin axis might be a promising prognostic index and a potential therapeutic target.
Hao C, Wang Z, Gu Y, Jiang WG, Cheng S. prognostic value of osteopontin splice variant-c expression in breast cancers: a meta-analysis. Biomed Res Int. 2016;2016:7310694. https://doi.org/10.1155/2016/7310694.
Xu YY, Zhang YY, Lu WF, Mi YJ, Chen YQ. Prognostic value of osteopontin expression in breast cancer: a meta-analysis. Mol Clin Oncol. 2015;3:357–62. https://doi.org/10.3892/mco.2014.480.
Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. https://doi.org/10.1111/j.1749-6632.2012.06750.x.
Gapstur SM, Drope JM, Jacobs EJ, Teras LR, McCullough ML, Douglas CE, et al. A blueprint for the primary prevention of cancer: targeting established, modifiable risk factors. CA Cancer J Clin. 2018;68:446–70. https://doi.org/10.3322/caac.21496.
Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–74. https://doi.org/10.1200/jco.2014.58.4680.
World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR). Diet, nutrition, physical activity and cancer: a global perspective. In: Continuous Update Project Expert Report 2018. London: WCRF International; 2018. wcrf.org/dietand-cancer/contents. Accessed May 24, 2019.
Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188–95. https://doi.org/10.1016/j.clbc.2012.12.002.
Birks S, Peeters A, Backholer K, O’Brien P, Brown W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13:868–91. https://doi.org/10.1111/j.1467-789X.2012.01010.x.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. https://doi.org/10.1056/NEJMoa1614362.
Weigl J, Hauner H, Hauner D. Can nutrition lower the risk of recurrence in breast cancer? Breast Care (Basel). 2018;13:86–91. https://doi.org/10.1159/000488718.
Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–9. https://doi.org/10.1007/s11695-018-3501-8.
Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? a systematic review. Obes Surg. 2017;27:3014–20. https://doi.org/10.1007/s11695-017-2901-5.
Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81. https://doi.org/10.1210/jc.2005-2248.
Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavalda JM, Hernandez M, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–8. https://doi.org/10.1007/s11695-013-1033-9.
Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. https://doi.org/10.1016/s1470-2045(09)70159-7.
Zhu J, Schott M, Liu R, Liu C, Shen B, Wang Q, et al. Intensive glycemic control lowers plasma visfatin levels in patients with type 2 diabetes. Horm Metab Res. 2008;40:801–5. https://doi.org/10.1055/s-0028-1082040.
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–19. https://doi.org/10.1016/j.diabres.2018.05.023.
Rahmani J, Manzari N, Thompson J, Gudi SK, Chhabra M, Naik G, et al. The effect of metformin on biomarkers associated with breast cancer outcomes: a systematic review, meta-analysis, and dose-response of randomized clinical trials. Clin Transl Oncol. 2019. https://doi.org/10.1007/s12094-019-02108-9.
Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schunemann HJ, et al. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2018;27:627–35. https://doi.org/10.1158/1055-9965.epi-17-0936.
Zhang ZJ, Yuan J, Bi Y, Wang C, Liu Y. The effect of metformin on biomarkers and survivals for breast cancer- a systematic review and meta-analysis of randomized clinical trials. Pharmacol Res. 2019;141:551–5. https://doi.org/10.1016/j.phrs.2019.01.036.
Shafiei-Irannejad V, Samadi N, Yousefi B, Salehi R, Velaei K, Zarghami N. Metformin enhances doxorubicin sensitivity via inhibition of doxorubicin efflux in P-gp-overexpressing MCF-7 cells. Chem Biol Drug Des. 2018;91:269–76. https://doi.org/10.1111/cbdd.13078.
Catalano S, Mauro L, Bonofiglio D, Pellegrino M, Qi H, Rizza P, et al. In vivo and in vitro evidence that PPARgamma ligands are antagonists of leptin signaling in breast cancer. Am J Pathol. 2011;179:1030–40. https://doi.org/10.1016/j.ajpath.2011.04.026.
Du R, Lin L, Cheng D, Xu Y, Xu M, Chen Y et al. Thiazolidinedione therapy and breast cancer risk in diabetic women: a systematic review and meta-analysis. Diabetes Metab Res Rev 2018;34. https://doi.org/10.1002/dmrr.2961.
Islam MM, Yang HC, Nguyen PA, Poly TN, Huang CW, Kekade S, et al. Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet. 2017;296:1043–53. https://doi.org/10.1007/s00404-017-4533-3.
Mansourian M, Haghjooy-Javanmard S, Eshraghi A, Vaseghi G, Hayatshahi A, Thomas J. Statins Use and risk of breast cancer recurrence and death: a systematic review and meta-analysis of observational studies. J Pharm Pharm Sci. 2016;19:72–81. https://doi.org/10.18433/j3202b.
Wu QJ, Tu C, Li YY, Zhu J, Qian KQ, Li WJ, et al. Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget. 2015;6:42988–3004. https://doi.org/10.18632/oncotarget.5557.
Lu L, Shi L, Zeng J, Wen Z. Aspirin as a potential modality for the chemoprevention of breast cancer: a dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget. 2017;8:40389–401. https://doi.org/10.18632/oncotarget.16315.
Zhong S, Chen L, Zhang X, Yu D, Tang J, Zhao J. Aspirin use and risk of breast cancer: systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomark Prev. 2015;24:1645–55. https://doi.org/10.1158/1055-9965.epi-15-0452.
Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–50. https://doi.org/10.1007/s10549-008-0228-6.
Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6:195–203. https://doi.org/10.1007/s13679-017-0263-x.
Wright CM, Moorin RE, Chowdhury EK, Stricker BH, Reid CM, Saunders CM, et al. Calcium channel blockers and breast cancer incidence: an updated systematic review and meta-analysis of the evidence. Cancer Epidemiol. 2017;50:113–24. https://doi.org/10.1016/j.canep.2017.08.012.
Sperati F, Vici P, Maugeri-Sacca M, Stranges S, Santesso N, Mariani L, et al. Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8:e69269. https://doi.org/10.1371/journal.pone.0069269.
Lazzeroni M, Gandini S, Puntoni M, Bonanni B, Gennari A, DeCensi A. The science behind vitamins and natural compounds for breast cancer prevention. Getting the most prevention out of it. Breast. 2011;20(Suppl 3):S36–41. https://doi.org/10.1016/s0960-9776(11)70292-2.
Zhang YF, Shi WW, Gao HF, Zhou L, Hou AJ, Zhou YH. Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PLoS One. 2014;9:e100044. https://doi.org/10.1371/journal.pone.0100044.
Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. Bmj. 2017;359:j4761. https://doi.org/10.1136/bmj.j4761.
Gianfredi V, Vannini S, Moretti M, Villarini M, Bragazzi NL, Izzotti A, et al. Sulforaphane and epigallocatechin gallate restore estrogen receptor expression by modulating epigenetic events in the breast cancer cell line MDA-MB-231: a systematic review and meta-analysis. J Nutrigenet Nutrigenomics. 2017;10:126–35. https://doi.org/10.1159/000480636.
Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–13. https://doi.org/10.1016/j.cytogfr.2013.10.001.
Catalano S, Leggio A, Barone I, De Marco R, Gelsomino L, Campana A, et al. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J Cell Mol Med. 2015;19:1122–32. https://doi.org/10.1111/jcmm.12517.
Leggio A, Catalano S, De Marco R, Barone I, Ando S, Liguori A. Therapeutic potential of leptin receptor modulators. Eur J Med Chem. 2014;78:97–105. https://doi.org/10.1016/j.ejmech.2014.03.048.
Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. https://doi.org/10.1186/bcr2321.
Otvos L Jr, Kovalszky I, Olah J, Coroniti R, Knappe D, Nollmann FI, et al. Optimization of adiponectin-derived peptides for inhibition of cancer cell growth and signaling. Biopolymers. 2015;104:156–66. https://doi.org/10.1002/bip.22627.
Bai J, Liao C, Liu Y, Qin X, Chen J, Qiu Y, et al. Structure-based design of potent nicotinamide phosphoribosyltransferase inhibitors with promising in vitro and in vivo antitumor activities. J Med Chem. 2016;59:5766–79. https://doi.org/10.1021/acs.jmedchem.6b00324.
Nimptsch K, Pischon T. Obesity Biomarkers, metabolism and risk of cancer: an epidemiological perspective. Recent Results Cancer Res. 2016;208:199–217. https://doi.org/10.1007/978-3-319-42542-9_11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gerasimos Socrates Christodoulatos, Nikolaos Spyrou, Jona Kadillari, Sotiria Psallida, and Maria Dalamaga declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Metabolism
GS Christodoulatos and N Spyrou have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Christodoulatos, G.S., Spyrou, N., Kadillari, J. et al. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr Obes Rep 8, 413–433 (2019). https://doi.org/10.1007/s13679-019-00364-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-019-00364-y