Abstract
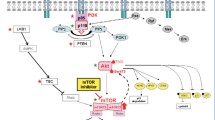
The mammalian (mechanistic) target of rapamycin (mTOR) is frequently activated in epithelial ovarian cancer and is regarded as an attractive therapeutic target. Preclinical investigations using mTOR complex 1 (mTORC1) inhibitors have demonstrated promising antitumor activity on ovarian cancer both in the setting of monotherapy and in combination with cytotoxic agents. Based on promising preclinical data, mTORC1 inhibitors are currently being evaluated in phase I/II trials in patients with ovarian cancer. In an effort to overcome resistance to mTORC1 inhibitors, the novel mTOR kinase inhibitors that inhibit both mTORC1 and mTORC2, or dual PI3K/mTOR inhibitor have recently been developed. In this report, we review the scientific rationale and evidence for the potential clinical benefits provided by mTOR inhibitors for patients with epithelial ovarian cancer.


Similar content being viewed by others
Abbreviations
- CCC:
-
Clear cell carcinoma
- SAC:
-
Serous adenocarcinoma
- mTOR:
-
mammalian target of rapamycin
- mTORC:
-
mTOR complex
- S6K-1:
-
Ribosomal S6 kinase-1
- 4E-BP1:
-
elF4E binding protein 1
- PI3K:
-
Phosphatidylinositol 3-kinase
- cisplatin:
-
cis-diamminedichloroplatinum
- PTEN:
-
Phosphatase and tensin homologue deleted on chromosome 10
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22.
Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–34.
Mabuchi S, Hisamatsu T, Kimura T. Targeting mTOR signaling pathway in ovarian cancer. Curr Med Chem. 2011;18:2960–8.
Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757–68. This study is the first to show the mechanism by which mTORC2 is activated.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo). 1975;28(10):727–32.
Mahalingam D, Sankhala K, Mita A, Giles FJ, Mita MM. Targeting the mTOR pathway using deforolimus in cancer therapy. Future Oncol. 2009;5(3):291–303.
Kolasa IK, Rembiszewska A, Janiec-Jankowska A, Dansonka-Mieszkowska A, Lewandowska AM, Konopka B, et al. PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecol Oncol. 2006;103(2):692–7.
Hashiguchi Y, Tsuda H, Inoue T, Berkowitz RS, Mok SC. PTEN expression in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2006;101(1):71–5.
Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11(8):2875–8.
Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61(20):7426–9.
Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–44.
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89(19):9267–71.
Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;6:212.
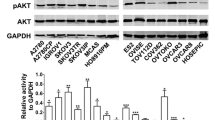
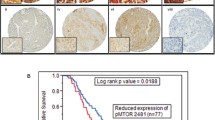
Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23(34):5853–7.
Mabuchi S, Kawase C, Altomare DA, Morishige K, Sawada K, Hayashi M, et al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res. 2009;15(17):5404–13.
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Shih IeM. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–601.
Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67(6):2408–13.
Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13(14):4261–70.
Jiang H, Feng Y. Hypoxia-inducible factor 1alpha (HIF-1alpha) correlated with tumor growth and apoptosis in ovarian cancer. Int J Gynecol Cancer. 2006;16 Suppl 1:405–12.
Schlosshauer PW, Li W, Lin KT, Chan JL, Wang LH. Rapamycin by itself and additively in combination with carboplatin inhibits the growth of ovarian cancer cells. Gynecol Oncol. 2009;114(3):516–22.
Mabuchi S, Hisamatsu T, Kawase C, Hayashi M, Sawada K, Mimura K, et al. The activity of trabectedin as a single agent or in combination with everolimus for clear cell carcinoma of the ovary. Clin Cancer Res. 2011;17(13):4462–73.
Huynh H, Teo CC, Soo KC. Bevacizumab and rapamycin inhibit tumor growth in peritoneal model of human ovarian cancer. Mol Cancer Ther. 2007;6(11):2959–66.
Kinross KM, Brown DV, Kleinschmidt M, Jackson S, Christensen J, Cullinane C, et al. In vivo activity of combined PI3K/mTOR and MEK inhibition in a Kras(G12D);Pten deletion mouse model of ovarian cancer. Mol Cancer Ther. 2011;10(8):1440–9.
Glaysher S, Bolton LM, Johnson P, Atkey N, Dyson M, Torrance C, et al. Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer. 2013;109(7):1786–94.
Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123(1):19–26. The only published result of phase II study of mTORC1 inhibitor.
NCT01196429. Clinical Trials.gov. A Service of the U.S. National Institute of Health. http://www.clinicaltrials.gov (Accessed 31 Mar 2014).
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81.
Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, et al. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21(15):5050–62.
Hisamatsu T, Mabuchi S, Matsumoto Y, Kawano M, Sasano T, Takahashi R, et al. Potential role of mTORC2 as a therapeutic target in clear cell carcinoma of the ovary. Mol Cancer Ther. 2013;12(7):1367–77. This study is the first to investigate the role of mTORC2 in the acquired resistance to mTORC1 inhibitor in ovarian cancer.
Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29(26):3733–44.
Ghadimi MP, Lopez G, Torres KE, Belousov R, Young ED, Liu J, et al. Targeting the PI3K/mTOR axis, alone and in combination with autophagy blockade, for the treatment of malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2012;11(8):1758–69.
Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–98.
NCT01936363. Clinical Trials.gov. A Service of the U.S. National Institute of Health. http://www.clinicaltrials.gov (Accessed 31 Mar 2014).
Acknowledgments
The authors thank Yuko Nishimura for providing secretarial assistance.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Seiji Mabuchi, Tomoyuki Sasano, Mahiru Kawano, Hiromasa Kuroda, and Tadashi Kimura declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Gynecological Cancer
Rights and permissions
About this article
Cite this article
Mabuchi, S., Sasano, T., Kawano, M. et al. Targeting mTOR Signaling in Ovarian Cancer. Curr Obstet Gynecol Rep 4, 11–17 (2015). https://doi.org/10.1007/s13669-014-0102-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-014-0102-y