Abstract
Purpose of Review
Dietary patterns that include polyphenols may help manage cardiometabolic risk factors. Pigmented rice contains phenolic acids and flavonoids that contribute to its antioxidant properties. This review examined the effect of polyphenol-containing pigmented rice on antioxidant status, lipid profile, glucose/insulin, blood pressure, and weight among adults. Four electronic databases including PubMed, ProQuest, EBSCOhost, and Google Scholar were systematically searched for relevant articles published in English since 2000, using PRISMA guidelines (PROSPERO registration: CRD42022358132). Two-staged screening resulted in the inclusion of seventeen (seven acute, ten chronic) randomized controlled trials. A random effects model was conducted on cardiometabolic outcomes reported in at least three studies.
Recent Findings
Acute intake increased plasma antioxidant activity and lowered postprandial glucose and insulin levels. Chronic consumption was associated with reductions in fasting glucose (WMD: -1.60 mg/dL; 95% CI:-3.05,-0.14, p = 0.03, k = 5, n = 349), weight (WMD: -0.23 kg, 95% CI: -0.44, -0.02, p = 0.03, k = 3, n = 182), and diastolic blood pressure (WMD: -1.39 mmHg, 95% CI: -2.21, -0.56, p = 0.001, k = 3, n = 185). No effect on total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, body mass index, and systolic blood pressure was found.
Summary
The consumption of pigmented rice may improve cardiometabolic risk factors. However, the small number of studies and differences in study design, including participants’ health status, form of rice utilized, and duration of intervention, support the need for more high-quality trials to further investigate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiometabolic risk factors such as dyslipidemia [1, 2], overweight and obesity [2, 3], hyperglycemia [1], and hypertension [1, 2, 4] influence the development of cardiovascular diseases (CVDs). Dyslipidemia, primarily elevated low-density lipoprotein (LDL) cholesterol, is recognized as an independent risk factor for developing CVDs [5], as when oxidized, induces regulatory T-cell apoptosis which promotes atherosclerosis [6]. Hyperglycemia promotes the formation of advanced glycation end products, which contribute to endothelial dysfunction [7], while obesity contributes to increased inflammation, insulin resistance, and dyslipidemia [8]. Elevated blood pressure (BP) increases the risk of vascular and cardiac damage [4].
Healthy diets that include polyphenols are shown to reduce cardiometabolic risk factors for CVDs [9]. Polyphenols are natural bioactive compounds synthesized by plants which contributes to their sensory and nutritional properties [10]. Polyphenols influence the development of CVDs by modulating inflammation, reducing LDL oxidation, and neutralizing free radicals [11,12,13]. Sources of polyphenols may vary depending on cultural and geographical factors [14]. Common sources are coffee and tea [15, 16], red wine [16, 17], fruits and vegetables, and cereals [15,16,17,18]. Rice (Oryza Sativa L), considered a staple in many countries [19•], contains several bioactive compounds, including polyphenols [20]. Specifically, pigmented rice which has colored bran fractions such as black, purple, and red [20], contains phenolic compounds, mainly ferulic acid, and flavonoids such as anthocyanins which influence its diverse colors [19•, 20]. Total anthocyanin content (TAC) and total phenolic content (TPC) are postulated to be correlated with antioxidant potential [21, 22]. Anthocyanins stabilize free radicals by donating an electron or hydrogen atom [23], and phenolic acids scavenge free radicals through hydrogen donation [24].
Animal models have identified several mechanisms through which pigmented rice may reduce cardiometabolic risk including the regulation of enzymes involved in metabolic pathways that influence lipid and glucose metabolism. Lower total cholesterol (TC), LDL, and triglycerides (TG) were attributed to a reduction in the expression of fatty acid synthase (FAS) [25, 26], an enzyme that catalyzes de novo synthesis of fatty acids, and an increase in carnitoyl transferase (CPT), which is essential for fatty acid oxidation [27]. Also demonstrated was increased levels of adiponectin [26], which is associated with improved insulin sensitivity, and reduced TG via increasing lipoprotein lipase activity [28]. Lastly, reduced adipose tissue and improved lipid levels were associated with down-regulation of peroxisome proliferator activated receptor—γ (PPAR-γ) [29]. Several human trials have recently been conducted to investigate the effect of pigmented rice consumption on lipids, glucose and body weight. However, the overall effect of pigmented rice on cardiometabolic risk factors has yet to be summarized. Thus, this review aimed to systematically review the body of evidence on the effect of pigmented rice on antioxidant status, cholesterol, glucose/insulin, BP, and weight in adults.
Materials and Methods
This review was conducted according to PRISMA guidelines, and the protocol is registered in the PROSPERO database (CRD42022358132).
Search Strategy
A core search strategy was first developed in PubMed by identifying MeSH terms for each concept (adult, pigmented rice, control, cholesterol, glucose, BP, phenolics, weight, and waist circumference (WC)), and using Boolean operators (See supplementary information). This was then used to systematically search for relevant studies in the following databases: PubMed, ProQuest, EBSCOhost, and Google Scholar (search completed October 25, 2022). In addition, online clinical trial registries (ClinicalTrials.gov, RIAT, Phil. Health Research Registry, and Australian New Zealand Clinical Trials Registry (ANZCTR)), reference lists of identified studies (pearling), and other sources of grey literature were searched to ensure all relevant studies were included. No limit on language was applied, but date of publication was limited from 2000 to the present as research outputs on the relationship between rice consumption and health increased from 2000 thereafter [20].
Eligibility Criteria/Study Selection
Table 1 presents the summarized eligibility criteria used in the present study. Randomized-controlled trials with (P) adult participants (> 18 years old) using (I) pigmented rice (red, purple, and/or black) in any form (including cooked, extract, powdered) as an intervention against a (C) control (placebo, white rice, brown rice, maltodextrin, or usual diet), and reporting at least one of the following outcomes (O): antioxidant status (total phenol index (TPI), total antioxidant/radical activity/capacity (TAC)), cholesterol (TC, LDL, high-density lipoprotein (HDL), TG), glucose/insulin (fasting blood glucose, glycated hemoglobin, insulin, insulin sensitivity), BP and anthropometry (weight, body mass index (BMI), WC/waist-hip ratio (W:H)) were included. Studies were excluded if they combined pigmented rice with other foods or intervention or did not compare to a control, such that the specific effects of pigmented rice could not be determined.
Studies were only eligible if published in full text in a peer-reviewed journal, in English (or with English translation). Animal and in vitro studies were excluded.
All studies were independently screened by title and abstract, then by full text by two reviewers (DMS; AMH, EVM, and RAR), and any disagreement was settled by a third reviewer (AMH, EVM, or RAR) using COVIDENCE software.
Data Extraction
Two reviewers independently extracted data (DMS; EVM and FPP) using a pilot-tested template, checked for discrepancies, and resolved by consensus. Extracted data included the following: author’s name, year of publication, country where the study was conducted, study design, study duration, blinding, study population characteristics (sex, age, co-morbidities, ethnicity), intervention (number of participants assigned and completed, type of rice, form, dosage, polyphenol content), comparator (number of participants assigned and completed, type, form, dosage), and pre- and post-intervention values (mean and standard deviation (SD), p-value)) for antioxidant status, cholesterol, glucose/insulin, BP, and weight.
Risk of Bias Assessment
The quality of included studies was assessed independently by two reviewers (DMS; EVM and RAR using the Cochrane Collaboration Risk of Bias Version 2 (RoB 2) tool, and a consensus resolved disagreements. Studies were assessed as low risk ( +), high risk (-) or some concerns (!), based on the following domains: (D1) bias arising from randomization process, (D2) bias due to deviations from intended interventions, (D3) bias due to missing outcome data, (D4) bias in measurement of outcome, and (D5) bias in selection of the reported result [30]. Crossover studies included assessment on bias arising from period carryover effects (DS).
Data Analysis
Chronic and acute study data were assessed separately for each outcome. Antioxidant activity in acute studies were analyzed by identifying the time points for initial increase of antioxidant activity, peak response, and duration of response. Pre-and post-intervention mean and standard deviation (SD) for TC, LDL, HDL, TG, glucose, BP, weight, and BMI were used to compute weighted mean differences (WMD) with their 95% confidence interval, and overall effect size for chronic studies. Standard deviations were calculated for studies that reported only standard error of the mean (SEM) using the formula: SD = SEM x square root (n). Studies without reported change and/or SD were calculated using the following formulas assuming a modest correlation coefficient (R) of 0.5 [31].
A random effects model was conducted on cardiometabolic outcomes reported in at least three studies using Review Manager version 5.4 [32], with a P-value of < 0.05 considered statistically significant. Heterogeneity was assessed using chi-squared test and I2 statistics. An I2 result of > 50% was considered substantial heterogeneity [33]. Forest plots were produced to present the summarized information on each study, heterogeneity, and overall effect size for each outcome. Due to the low number of studies included in the analyses, funnel plots were not generated.
Quality of evidence for each outcome was rated high, moderate, low, or very low based on the following criteria: (1) risk of bias, (2) inconsistency of results, (3) indirectness of evidence, (4) imprecision, and (5) publication bias [34], using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria [35].
Results
Study Selection
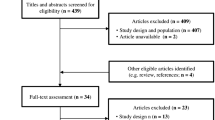
Figure 1 presents the flow of study selection based on PRISMA guidelines [36]. A total of 6,184 studies from the four databases and 40 from additional searches were identified. Duplicates were removed using a reference manager (Mendeley). A total of 4,537 studies were imported to COVIDENCE for screening. A total of 42 studies were identified for full-text review, of which 26 were excluded based on the following reasons: wrong intervention (n = 20), non-RCT (n = 3), wrong outcomes (n = 1), no English translation (n = 1), and with co-intervention (n = 1). Seventeen eligible studies were included in the systematic review.
Study Characteristics
Characteristics of the included studies are presented in Tables 2 and 3. Of the 17 studies, seven were acute and ten were chronic interventions. Eight studies used a cross-over design [37,38,39,40,41, 42••, 43, 44], and nine studies were parallel [45, 46, 47•, 48,49,50,51,52,53].
Most of the studies were from Asia, with five conducted in Thailand [38, 39, 41, 42••, 52], four in South Korea [45, 46, 47•, 48], two in Indonesia [50, 51], and one in Malaysia [43], Japan [49], and China [53], while others were from United Kingdom [37], Italy [44], and Australia [40]. Participants ranged in age from 18–75 years, were predominantly healthy [37, 38, 41, 42••, 43, 44, 49, 50], or with overweight/obesity [39, 47•, 48], Metabolic Syndrome [46, 51], CVD [53], memory impairment [45], or unspecified co-morbidities [52].
All acute studies were a cross-over design with red [40, 43], purple [39,40,41, 42••], and black [44] rice in various forms, including cooked [40, 42••, 43, 44], extract incorporated in sugary beverage [39] and yogurt [38], and powder used in bread [41]. These were compared against controls including rice (brown, white, basmati and jasmine), wheat bread, sucrose, and plain yogurt. All chronic studies used black rice but varied in form, such as those with giant embryo [46], in powder [48, 52, 53], extracts [45, 47•], cooked [49, 50], or incorporated into a snack bar [51, 54]. Three studies did not report a matched control [50,51,52]; other studies used rice (polished and white), maltodextrin, and cellulose. Duration of intervention for chronic studies was 4 [50, 51], 6 [48], 12 [45, 47•, 49], 24 [52, 53] weeks.
The polyphenol content of the pigmented rice used were reported in some studies as total polyphenols [44], flavonoids [44], anthocyanins [38, 39, 44, 49] and phenolics [43], with several including the specific content for phenolic acid (ferulic acid [49]), and anthocyanin such as cyanidin-3-glucoside [37,38,39, 45, 54], and peonidin-3-glucoside [37,38,39, 54]. In some studies, polyphenol content was derived from other studies [46, 50, 53] or not specified [40, 41, 42••, 47•, 48, 51].
Assessment of Risk of Bias
Figure 2 presents the results of the risk of bias assessment for parallel studies. Four studies were assessed as having some concerns attributed to the randomization process [48, 50,51,52]. Despite only two studies providing a complete discussion on how randomization was carried out [45, 47•] and three providing information on blinding [45, 47•, 53], all studies provided details on baseline characteristics of participants which assisted with assessing bias arising from the randomization process (D1). All studies, except for Syarief et al. [51] provided details on the sham intervention provided for the control which aids in assessing bias arising from deviations from intended intervention (D2). All study outcomes are considered observer reported outcomes not involving judgement which are not likely to be affected by knowledge of intervention received by the participants [31].
Figure 3 presents the results of the risk of bias assessment for crossover studies. Two of eight studies were assessed as having some concerns as they did not provide detailed descriptions of how randomization was done. Most studies (n = 6) reported at least a one-week wash out period in between treatment groups which was considered sufficient for minimizing carry-over effects.
Effect on Antioxidant Status
Nine studies (seven acute and two chronic) investigated the effect of pigmented rice consumption on antioxidant status [38,39,40,41, 42••, 43, 44, 48, 53]. Of the acute studies, three used Riceberry, a rice cultivar with deep-purple pigment [38, 39, 41], three provided black rice [44, 48, 53], and one study investigated both purple and red rice [40]. All acute studies reported an increase in antioxidant activity after 30 min of ingestion compared to control (brown rice, wheat bread, sucrose, or yogurt). However, peak antioxidant activity varied among studies, ranging from 30 to 360 min after ingestion (see Table 4). Duration of antioxidant activity lasted from 180 min [38, 41, 44] after ingestion, to as long as 240 [40] or 360 [39] minutes. Four acute studies demonstrated significant increases in several measures of antioxidant activity compared to control at various time points [38, 39, 41, 44], and one study reported a significant increase in FRAP compared to baseline [40].
Wang et al. [53] measured antioxidant activity after six months of intervention with black rice fraction among CHD patients. They demonstrated significantly higher FRAP activity compared to control (1.29 ± 2.96 103 u/L vs. -0.61 ± 1.69 103 u/L; p < 0.01) but no differences in superoxide dismutase (SOD). Similarly, Kim et al. [48] also showed no effect on SOD, but observed significantly higher glutathione peroxidase among women with obesity who consumed black rice meal replacements for six weeks compared to white rice meal replacements (15.36 ± 5.63 U/g Hb vs. 3.52 ± 5.41 U/g Hb, p < 0.05).
Effect on Lipids
All studies (n = 8, all chronic) that evaluated changes in TC, LDL, HDL, and TG were included in the meta-analysis. Significant heterogeneity can be observed among the pooled studies for TC (I2 = 67%), LDL (I2 = 80%), and TG (I2 = 89%). Pigmented rice consumption did not improve TC (WMD -2.05 mg/dL; 95% CI:-8.24,4.14, p = 0.52, n = 509, Fig. 4a), LDL (WMD -2.32 mg/dL; 95% CI:-9.21,4.58, n = 498, p = 0.51, Fig. 4b), HDL (WMD = -0.72 mg/dL; 95% CI:-1.44,0.01, p = 0.05, n = 507, Fig. 4c), or TG (WMD = 2.02 mg/dL; 95% CI:-15.83,19.87, p = 0.82, n = 507, Fig. 4d) compared to control.
Effect on Glucose
Eleven studies reported glucose as an outcome (five acute and six chronic intake). All data for postprandial glucose and insulin were reported in line graphs, which limited data extraction (only one of the contacted authors provided data). Only the chronic studies provided sufficient data for meta-analysis.
The majority of acute studies reported significantly lower postprandial glucose levels compared to control (plain yogurt, sucrose, wheat bread, white rice) [38, 39, 41, 42••] starting at 30 min up to 120 min after ingestion (Table 4). Additionally, three out of five studies that measured postprandial insulin reported significantly lower insulin levels compared to their control (sucrose, wheat bread, polished rice) [39, 41, 49] ranging from 15 min up to 120 min after ingestion (Table 4). Compared to control, chronic pigmented rice consumption significantly lowered fasting glucose (WMD = -1.60 mg/dL; 95% CI: -3.05, -0.14, I2 = 18%, p = 0.03, n = 349, Fig. 5).
Effect on Blood Pressure
Three chronic studies included BP as an outcome [45, 46, 47•]. Significant improvements were observed for diastolic BP only (WMD = -1.39 mmHg, 95% CI: -2.21, -0.56, I2 = 0%, p = 0.001, n = 185, Fig. 6b).
Effect on Weight and Body Mass Index
Three chronic studies included weight and BMI as outcomes [46, 47•, 48]. Pigmented rice consumption was associated with significant reductions in weight (WMD = -0.23 kg, 95% CI: -0.44, -0.02, I2 = 0%, p = 0.03, n = 182, Fig. 7a), but not BMI (WMD = -0.24 kg/m2, 95% CI: -0.59, 0.11, I2 = 82, p = 0.17, n = 182, Fig. 7b).
GRADE
The certainty assessment for each outcome is presented in Table 5. All outcomes were assessed to have no serious risk of bias. However, TC, LDL, TG, Glucose, and SBP have serious concerns for inconsistency and indirectness attributed to differences in the study population, and delivery of the intervention. Lastly, imprecision was assessed as serious for TC, LDL, BMI, weight, and DBP, and very serious for HDL, TG, and SBP.
Discussion
This study systematically reviewed the effect of pigmented rice consumption on antioxidant status and cardiometabolic risk factors in adults. Meta-analysis of chronic intake studies demonstrated significant reductions in glucose, weight, and diastolic BP, but no significant effects on TC, LDL, TG, HDL, BMI, or systolic BP. All acute studies included in the review demonstrated that pigmented rice consumption increases antioxidant activity 30 min after ingestion and is sustained for at least 180 min. Moreover, chronic consumption of pigmented rice (12–24 weeks) was shown to increase total antioxidant activity compared to control [48, 53]. This finding may explain the role of pigmented rice in improving blood pressure by reducing oxidative stress which promotes endothelial dysfunction [55]. Reactive oxygen species decrease nitric oxide production and promote cellular damage, resulting to inflammation, vasoconstriction, vascular lesion and ultimately atherosclerosis and CVDs [56].
The antioxidant activity of pigmented rice is attributed to its polyphenol content such as phenolic acids that can stabilize electrons, and flavonoids (including anthocyanins) that have the ability to donate electrons and stop chain reactions [20]. Various studies have shown pigmented rice promotes radical scavenging activity (DPPH) [44], has reducing power (FRAP) [38, 39, 41, 53], ability to inhibit radical induced oxidation (ORAC [38] and ABTS [44]), ability to neutralize radical cation (TEAC) [38,39,40], and reduces oxidative stress (MDA) [38,39,40,41]. Despite having low bioavailability, once absorbed in the gut anthocyanins are metabolized in the liver, secreted and reabsorbed in the enterohepatic circulation resulting in molecular intermediates that contribute to their biologic actions [57].
The meta-analysis showed significant beneficial effects on glucose, weight, and diastolic BP, but not cholesterol (TC, LDL, HDL, TG), BMI, or systolic BP. Black rice anthocyanins may help reduce glucose levels by delaying carbohydrate absorption through inhibition of α-amylase and α-glucosidase [58]. This can be seen in the acute intake studies where the majority demonstrated significantly lower postprandial glucose and insulin levels at various timepoints compared to the control. This may imply that pigmented rice consumption may help maintain lower blood glucose levels post meal. However, it should be noted that despite significant reductions in fasting blood glucose levels in chronic studies, the magnitude of effect was small and unlikely to be considered clinically meaningful. Participants in this analysis had normal blood glucose levels at baseline; it is possible that greater benefit may be seen in persons with elevated glucose levels. Additionally, polyphenols may assist with managing obesity through increased energy expenditure, appetite suppression, and regulation of lipid metabolism [59]. Several mechanisms of action have been postulated for how pigmented rice consumption may reduce lipid levels. These include regulation of fatty acid synthesis [25, 26], transcription factors [29], and lipid metabolism [26]. However, while most studies included in this review reported reductions in lipids, these were not statistically different to the control, and collectively this meta-analysis did not show any benefit on lipids. Manach et al. [60] argues that doses in animal studies are often higher than what human tissue may be exposed to. Additionally, participants in many of the studies included in this review had normal TC, LDL, TG, and glucose levels, as opposed to most animal studies which induce hyperlipidemia or hyperglycemia.
Moreover, considerable differences in study characteristics were noted. While all studies in the meta-analysis used black rice, they varied in form (cooked, extract, fermented, glutinous) and dosage (15 mg to 320 mg anthocyanin). Phenolic acids, mostly ferulic acid, and anthocyanins which are mostly cyanidin 3-O-glucodside and peonidin 3-O- glucoside, are highly concentrated in the bran [20]. Further, different rice forms (extract, with giant embryo, glutinous) and effect of processing (cooked, fermented, incorporated to a snack bar) may contribute to differences in concentration and bioavailability of polyphenols. Black sticky rice was reported to have higher antioxidant activity compared with red, and black rice [19•]. Germination of rice also increase antioxidant activity, total phenolics and flavonoids [61], while fermentation of grains is hypothesized to increase bioavailability of phenolic compounds [62]. The study of Fauziyah et al. [50] which utilized fermented glutinous black rice reported the greatest reduction in LDL among all studies included in this review, which was significantly lower compared to the control. Most of the chronic studies matched their control with the intervention, either using white/polished rice in the same form as the intervention (i.e., whole, powdered, meal replacement mix) or using placebo/maltodextrin capsules identical to the extracts administered to the intervention group. However, some studies did not report the specific control used [50, 51], as noted in the assessment of risk of bias, or did not attempt to match the rice form given to the intervention group [52]. Lastly, included studies differed in duration of intervention, and presence or absence of co-intervention. Apart from the rice, one study also provided diet counselling to both groups [50]; one study induced energy restriction in both groups [48]. Seesen et al. [52], provided health education to the control in lieu of the intervention. Due to the limited number of studies, subgroup analyses could not be conducted to determine whether these differences impacted on the cardiometabolic outcomes. Altogether, these differences influence the certainty of evidence of the outcomes. Therefore, further randomized controlled human clinical trials are warranted to support the clinical value of pigmented rice consumption for reducing cardiometabolic risk factors.
The present meta-analysis has some limitations that should be noted. First, some of the included studies have some concerns based on Cochrane Collaboration’s Risk of Bias, specifically in relation to how randomization was carried out. Secondly, no meta-analysis was conducted on antioxidant status, or postprandial glucose and insulin due to availability of data from included studies. Outcomes with meta-analyses have heterogeneity (TC, LDL, TG, BMI, and systolic BP) which is likely attributed to differences in study design.
Conclusion
This systematic review showed that acute intake of pigmented rice increases antioxidant activity and lowers postprandial glucose and insulin levels. Meta-analysis demonstrated significant reductions in glucose, weight, and diastolic BP following chronic pigmented rice consumption, but no significant effects on TC, LDL, TG, HDL, BMI, or systolic BP. More high quality randomized controlled trials are warranted to further investigate the effect of pigmented rice consumption on cardiometabolic risk factors in adults; additional benefit may be observed in those with established clinical conditions such as dyslipidemia, pre-diabetes/diabetes, and hypertension.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Wilson PWF, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 2008;117(23):3031–8. https://doi.org/10.1161/CIRCULATIONAHA.107.738732.
Rosolova H, Nussbaumerova B. Cardio-metabolic risk prediction should be superior to cardiovascular risk assessment in primary prevention of cardiovascular diseases. EPMA J. 2011;2(1):15–26. https://doi.org/10.1007/S13167-011-0066-1.
Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113(6):898–918. https://doi.org/10.1161/CIRCULATIONAHA.106.171016.
Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–92. https://doi.org/10.1161/HYPERTENSIONAHA.119.14240.
de Oliveria Barbosa Rosa C, dos Santos CA, Leite JIA, Caldas APS, Bressan J. Impact of nutrients and food components on dyslipidemias: what is the evidence? Adv Nutr. 2015;6(6):703–11. https://doi.org/10.3945/AN.115.009480.
Amersfoort J, Schaftenaar FH, Douna H, et al. Diet-induced dyslipidemia induces metabolic and migratory adaptations in regulatory T cells. Cardiovasc Res. 2021;117(5):1309–24. https://doi.org/10.1093/CVR/CVAA208.
Meza CA, La Favor JD, Kim DH, Hickner RC. Endothelial dysfunction: is there a hyperglycemia-induced imbalance of NOX and NOS? IJMS. 2019;20(15):3775. https://doi.org/10.3390/IJMS20153775.
Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021. https://doi.org/10.1161/CIR.0000000000000973.
Grosso G, Stepaniak U, Micek A, et al. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur J Nutr. 2018;57(4):1535–44. https://doi.org/10.1007/S00394-017-1438-7.
Bertelli A, Biagi M, Corsini M, Baini G, Cappellucci G, Miraldi E. Polyphenols: from theory to practice. Foods. 2021;10(11):2595. https://doi.org/10.3390/FOODS10112595.
Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: A mini-review. Front Nutr. 2018;5:87. https://doi.org/10.3389/FNUT.2018.00087.
Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10(2):514–28. https://doi.org/10.1039/C8FO01997E.
Koch W. Dietary polyphenols—important on-Nuntrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients. 2019;11(5):1039. https://doi.org/10.3390/NU11051039.
Del Bo’ C, Bernardi S, Marino M, et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients. 2019;11(6):1355. https://doi.org/10.3390/NU11061355.
Huang Q, Braffett BH, Simmens SJ, Young HA, Ogden CL. Dietary polyphenol intake in US adults and 10-year trends: 2007–2016. J Acad Nutr Diet. 2020;120(11):1821–33. https://doi.org/10.1016/j.jand.2020.06.016.
Zamora-Ros R, Knaze V, Rothwell JA, et al. Dietary polyphenol intake in Europe: The European prospective investigation into cancer and nutrition (EPIC) study. Eur J Nutr. 2016;55(4):1359–75. https://doi.org/10.1007/S00394-015-0950-X.
Karam J, del Mar Bibiloni A, Tur J. Polyphenol estimated intake and dietary sources among older adults from Mallorca Island. PLoS ONE. 2018;13(1):e0191573. https://doi.org/10.1371/JOURNAL.PONE.0191573.
Taguchi C, Fukushima Y, Kishimoto Y, et al. Estimated dietary polyphenol intake and major food and beverage sources among elderly Japanese. Nutrients. 2015;7(12):10269–81. https://doi.org/10.3390/NU7125530.
• Callcott ET, Santhakumar AB, Luo J, Blanchard CL. Therapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J Appl Biomed. 2018;16(4):255–62. https://doi.org/10.1016/J.JAB.2018.03.001. This review article comprehensively discusses the bioactive compounds and mechanisms of action of rice-derived polyphenols.
Goufo P, Trindale H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2(2):75–104. https://doi.org/10.1002/fsn3.86.
Francavilla A, Joye IJ. Anthocyanins in whole grain cereals and their potential effect on health. Nutrients. 2020;12(10):2922. https://doi.org/10.3390/NU12102922.
Bulatao RM, Samin JPA, Huliganga RC, Tubera RP, Feliciano MAM, Ortinero CV. Phytochemical properties, antioxidant activities, and cytotoxicity of ethanolic bran extracts from Philippine pigmented rice cultivars. Phillip Agric Scientist. 2020;103(4):10.
Garcia C, Blesso CN. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic Biol and Med. 2021;172:152–66. https://doi.org/10.1016/J.FREERADBIOMED.2021.05.040.
Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst). 2019;24:e00370. https://doi.org/10.1016/J.BTRE.2019.E00370.
Lo LMP, Kang MY, Yi SJ, Chung SI. Dietary supplementation of germinated pigmented rice ( Oryza sativa L.) lowers dyslipidemia risk in ovariectomized Sprague-Dawley rats. Food Nutr Res. 2016;60(1):30092. https://doi.org/10.3402/FNR.V60.30092.
Chung SI, Kang MY. Oral administration of germinated, pigmented, giant embryo rice (Oryza sativa L. cv. Keunnunjami) extract improves the lipid and glucose metabolisms in high-fat diet-fed mice. Oxid Med Cell Longev. 2021;2021:1–9. https://doi.org/10.1155/2021/8829778.
Wang M, Wang K, Liao X, et al. Carnitine palmitoyltransferase system: a new target for anti-inflammatory and anticancer therapy? Front Pharmacol. 2021;12:760581. https://doi.org/10.3389/FPHAR.2021.760581.
Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. IJMS. 2019;20(5):1190. https://doi.org/10.3390/IJMS20051190.
Kim HW, Lee AY, Yeo SK, et al. Metabolic profiling and biological mechanisms of body fat reduction in mice fed the ethanolic extract of black-colored rice. Food Res Int. 2013;53(1):373–90. https://doi.org/10.1016/J.FOODRES.2013.05.001.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from https://training.cochrane.org/handbook.
Review Manager (RevMan) [Computer program] Version 5.4 The Cochrane Collaboration. RevMan. Published 2020. https://training.cochrane.org/online-learning/core-software/revman. Accessed 19 Sept 2022.
Deeks J, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. Published July 2019. https://training.cochrane.org/handbook/archive/v6/chapter-10. Accessed 19 Sept 2022.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from https://guidelinedevelopment.org/handbook.
McMaster University, Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Published online 2022. https://gradepro.org/. Accessed 17 Jan 2023.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 Statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi.org/10.1186/S13643-021-01626-4.
Aboufarrag H, Hollands WJ, Percival J, Philo M, Savva GM, Kroon PA. No effect of isolated anthocyanins from bilberry fruit and black rice on LDL cholesterol or other biomarkers of cardiovascular disease in adults with elevated cholesterol: A randomized, placebo-controlled, cross-over trial. Mol Nutr Food Res. 2022;66(21):2101157. https://doi.org/10.1002/MNFR.202101157.
Anuyahong T, Chusak C, Thilavech T, Adisakwattana S. Postprandial effect of yogurt enriched with anthocyanins from Riceberry rice on glycemic response and ntioxidant Capacity in healthy adults. Nutrients. 2020;12(10):2930. https://doi.org/10.3390/NU12102930.
Anuyahong T, Chusak C, Adisakwattana S. Riceberry rice beverage decreases postprandial glycemic response, inflammatory markers and antioxidant status induced by a igh-carbohydrate and moderate-at meal in overweight and obese men. Food Funct. 2022;13(2):834–45. https://doi.org/10.1039/D1FO03169D.
Callcott ET, Blanchard CL, Snell P, Santhakumar AB. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019;10(12):8016–25. https://doi.org/10.1039/C9FO02261A.
Chusak C, Pasukamonset P, Chantarasinlapin P, Adisakwattana S. Postprandial glycemia, insulinemia, and antioxidant status in healthy subjects after ingestion of bread made from anthocyanin-rich Riceberry rice. Nutrients. 2020;12(3):782. https://doi.org/10.3390/NU12030782.
•• Muangchan N, Khiewvan B, Chatree S, et al. Riceberry Rice (Oryza sativa L.) slows gastric emptying and improves the postprandial glycaemic response. Br J Nutr. 2022;128(3):424–32. https://doi.org/10.1017/S0007114521003494. This study evaluated the effect of pigmented rice on postprandial glycemia. Findings of the study suggest that pigmented rice consumption may help glycemic control by slowing gastric emptying rate.
Se CH, Chuah KA, Mishra A, Wickneswari R, Karupaiah T. Evaluating crossbred red ice variants for postprandial glucometabolic responses: a comparison with commercial varieties. Nutrients. 2016;8(5):308. https://doi.org/10.3390/NU8050308.
Vitalini S, Sardella A, Fracassetti D, et al. Polyphenol bioavailability and plasma antiradical capacity in healthy subjects after cute intake of pigmented rice: a crossover randomized controlled clinical trial. JCM. 2020;9(10):3209. https://doi.org/10.3390/JCM9103209.
Joo SH, Hahn C, Lim HK, Yoon KD, Yoon SH, Lee CU. An exploration of the Oryza sativa L. Cyanidin-3-glucoside on the cognitive function in older adults with subjective memory impairment. Psychiatry Investig. 2019;16(10):759–65. https://doi.org/10.30773/PI.2019.06.17.
Joo NS, Han SI, Kim KN, et al. Black rice with giant embryo ameliorates seum C-reactive protein in adults with metabolic syndrome. J Clin Biochem Nutr. 2020;67(3):344–8. https://doi.org/10.3164/JCBN.20-72.
• Jung AJ, Sharma A, Lee SH, Lee SJ, Kim JH, Lee HJ. Efficacy of black rice extract on obesity in obese postmenopausal women: A 12-week randomized, double-blind, placebo-controlled preliminary clinical trial. Menopause. 2021;28(12):1391–9. https://doi.org/10.1097/GME.0000000000001862. This study evaluated the efficacy of black rice extract on body fat and other cardiometabolic markers among obese postmenopausal women after 12 weeks of intervention. The study provides preliminary findings on the possible therapeutic benefits of pigmented rice.
Kim JY, Kim JH, Lee DH, Kim SH, Lee SS. Meal replacement with mixed rice is more effective than white rice in weight control, while improving antioxidant enzyme activity in obese women. Nutr Res. 2008;28(2):66–71. https://doi.org/10.1016/J.NUTRES.2007.12.006.
Nakamura S, Ikeuchi T, Araki A, et al. Possibility for prevention of Type 2 Diabetes Mellitus and dementia using three kinds of brown rice blends after high-pressure treatment. Foods. 2022;11(6):818. https://doi.org/10.3390/FOODS11060818.
Fauziyah RN, Fitriani N, Surmita S. Effect of fermented glutinous black rice on LDL cholesterol levels. J Ris Kesehat Poltekkes Depkes Bandung. 2020;12(1):10.
Syarief O, Fauziyah RN, Suparman S, Pramintarto G, Hendriyani H. The efficacy of fermented glutinous black rice (fgbr) snack to improve lipid profile among dyslipidemia subjects: a novel finding. IMJ. 2020;25(08):9.
Seesen M, Semmarath W, Yodkeeree S, et al. Combined black rice germ, bran supplement and exercise intervention modulate aging biomarkers and improve physical performance and lower-body muscle strength parameters in aging population. IJERPH. 2020;17(8):2931. https://doi.org/10.3390/IJERPH17082931.
Wang Q, Han P, Zhang M, Xia M, Zhu H, Ma J, Hou M, Tang Z, Ling W. Supplementation of black rice pigment fraction improves antioxidant and anti-inflammatory status in patients with coronary heart disease. Asia Pac J Clin Nutr. 2007;16(Suppl 1):295–301. PMID: 17392122.
Hongu N, Kitts DD, Zawistowski J, et al. Pigmented rice bran and plant sterol combination reduces serum lipids in overweight and obese adults. J Am Coll Nutr. 2014;33(3):231–8. https://doi.org/10.1080/07315724.2013.869772.
Potì F, Santi D, Spaggiari G, Zimetti F, Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. IJMS. 2019;20(2):351. https://doi.org/10.3390/IJMS20020351.
Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. ATVB. 2003;23(2):168–75. https://doi.org/10.1161/01.ATV.0000051384.43104.FC.
Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25(17):3809. https://doi.org/10.3390/MOLECULES25173809.
Mbanjo EGN, Kretzschmar T, Jones H, et al. The genetic basis and nutritional benefits of pigmented rice grain. Front Genet. 2020;11:229. https://doi.org/10.3389/FGENE.2020.00229.
Singh M, Thrimawithana T, Shukla R, Adhikari B. Managing obesity through natural polyphenols: a review. Future Foods. 2020;1–2:100002. https://doi.org/10.1016/J.FUFO.2020.100002.
Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16(1):77–84. https://doi.org/10.1097/00041433-200502000-00013.
do Nascimento LÁ, Abhilasha A, Singh J, Elias MC, Colussi R. Rice germination and its impact on technological and nutritional properties: A review. Rice Sci. 2022;29(3):201–15. https://doi.org/10.1016/J.RSCI.2022.01.009.
Adebo OA, Gabriela M-M. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules. 2020;25(4):927. https://doi.org/10.3390/MOLECULES25040927.
Acknowledgements
Authors would like to thank Riza A. Ramos, Ph.D. of Philippine Rice Research Institute for assisting with the full text screening and risk of bias assessment; and Ms. Florimae P. Paimalan for assisting with data extraction and assessing the certainty of evidence.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions The current study did not receive any specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
DMS and AMH designed and finalized the research; DMS and EVM conducted the systematic search; DMS, AMH and EVM performed the title and abstract screening; DMS, and EVM conducted the full text review screening. DMS and EVM extracted the data; DMS and AMH analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest for the current study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mendoza-Sarmiento, D., Mistades, E.V. & Hill, A.M. Effect of Pigmented Rice Consumption on Cardiometabolic Risk Factors: A Systematic Review of Randomized Controlled Trials. Curr Nutr Rep 12, 797–812 (2023). https://doi.org/10.1007/s13668-023-00496-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00496-7