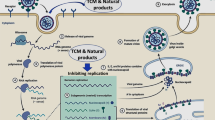
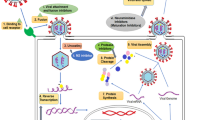
Abstract
The search for a potent anti-coronavirus therapy for severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2) remains an overwhelming task since the outbreak of COVID-19. It is more evident that most of the existing antiviral and immune-boosting drugs are non-promising and ineffective for the treatment of coronavirus infected patients while the safety of a few drugs/vaccines that have demonstrated high potential remains unclear. With daily records of confirmed infectious cases across the world, it is crucial to emphasize the need for repurposed therapies with a validated ethnomedicinal base focused on well-known active medicines with traceable biochemical, pharmacological and safety profiles for viral infection management. In the present study, recent literature on Artemisia and Artemisia-based products for the management of COVID-19 are reviewed. Artemisia-based products have demonstrated a broad spectrum of biological ability including antiviral properties. Besides its antiviral activity, Artemisia annua have shown to contain appreciable amounts of minerals such as zinc, gallium and selenium among others.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A viable alternative therapy in the management of COVID-19 is phytomedicine. The use of traditional medicine has a long history in nature. Since antiquity, plant resources have contributed immensely to health and medicine. The various secondary metabolites they possess have made them medically useful. Herbal medicine is predominantly utilized in countries like China, India, Egypt and many others on the African continent for the treatment of several (mild and severe) ailments and infectious diseases, including coronavirus infections (Jin et al. 2020; Yang et al. 2020). The novel coronavirus (SARS-CoV-2) is an etiological agent of COVID-19 (WHO 2020c). Although the statistics of the disease-related morbidity and mortality have reduced, there remain several infectious cases across the world aside from other existing asymptomatic cases (WHO 2020a, 2020b).
To date, there is yet no effective, approved therapy or vaccine for the treatment of the COVID-19. This, therefore, stresses the need to find an effective and safe approach for the management of infected patients. Most notably, the aurora of natural product-based drug discovery is emerging and boosting body immunity with validated ethnomedicine remains an innovative therapeutic strategy (Tong and Deng 2020).
The emergence of Artemisia annua (A. annua) and its derivatives as effective therapy against malaria pathogen, Plasmodium falciparum, has led to transverse researches exploring newer and diverse pharmaceutical potentials of A. annua extracts and its artemisinin derivatives (Liu et al. 2019; Zyad et al. 2018). Notably, the outbreak of COVID-19 pandemic, its global human-to-human transmission curve, and the resultant mortality rate have beamed attention on the viability, safety, and efficacy of Artemisia and its derivatives as a potential therapeutic drug for the treatment of SARS-COV-2.
Very recently, some African countries have reportedly claimed that the anecdotal use of an extract of A. annua is efficacious for COVID-19 management, albeit with no scientific evidence demonstrated. In ethnobotanical practice, the whole plant is commonly used for treating malaria, cough, and cold (Nigam et al. 2019). A practice that could likely be based on the recommendation of artemisinin as a component of ACT (artemisinin-combination based therapies) for malaria by the World Health Organization (WHO 2015). In a recent study, Boukhatem and Setzer (2020) reviewed the antiviral potentials of some aromatic herbs, medicinal plant-derived essential oils, and phytochemicals including Artemisia against various coronaviruses. The isolated pure compounds are known for their immune-modulating and pro-inflammatory host response enhancing properties. It should be noted that Artemisia plants contain several essential oils and bioactive chemical components (Martínez et al. 2012), which broadens their biological activity beyond antimalarial function. While they are widely spread across various continents (particularly in Asia and Africa), they possess an appreciable amount of nutritional values and several health benefits (Brisibe et al. 2009; Nigam et al. 2019).
Remarkably, the broad range of bioactive components in A. annua forms a frontline basis of its adoption as an antiviral drug and therapeutic option against coronavirus infection. Interestingly, it has a high accumulation of minerals (Table 1) as reported by Poisson-Benatouil (2020) including potassium, essential amino acids and low sodium content (Ferreira; IqbalHussain and Khattak 2011). Also, there have been some indications to repurpose Artemisinin, a sesquiterpene lactone derivative of Artemisia (Fig. 1a) as well as other semi-synthetic derivatives (principally Artemether, Arteether and Artesunate) (Fig. 1b) to stem the debilitating effect of COVID-19. Consequently, a detailed review is significantly needed to give an overview of the anti-coronavirus effect exhibited by Artemisia and Artemisia-based products as well as to motivate further research on the drugs especially as it has been projected that COVID-19 infection may remain for several years. This paper therefore reviews recent literature on the biochemical, pharmacological and safety profiles of Artemisia and Artemisia-based products in the management of COVID-19.
Research methodology
This review presents an overview of scientific contributions on Artemisia and Artemisia-based products in the management of COVID-19 including clinical trials and safety information. Scientific articles and reports on Artemisia and Artemisia-based products for the management of SARS-CoV-2 were carefully accessed from online databases such as Google Scholar, PubMed, NCBI, Researchgate COVID-19, ScienceDirect, SciFinder, Web of Science and other scientific databases on COVID-19 such as WHO situation reports on COVID, US National Library of Medicine and Chinese clinical trial registry were also accessed using the keywords; Artemisia, Artemi, SARS-CoV-2, COVID-19. Also, Chem-Draw Ultra 8.0 (Cambridge Soft, 100 Cambridge Park Drive, Cambridge MA 02140) was used for drawing chemical structures.
Origin and bioavailability of Artemisia
The genus Artemisia commonly known as Sagebrush, wormwood, and Sagewort belongs to the largest flowering plants family Asteraceae. This genus is represented by small herbs and shrubs of vascular plants, annual or perennial, with a strong and pleasant aromatic smell, and consists of about 500 species distributed across Africa, Asia, Australia, Europe, Central and South America with 186 species (82 of them endemics) in China. They can survive in the temperate climates of both hemispheres, usually in dry or semiarid and wetland habitats, while exhibiting various life forms (Martín et al. 2003; Wright 2001; Yu and Zhong 2001). Also, they are largely cosmopolitan, inhabiting from sea level, and often landscape dominating. It is distributed across all continents except in Antarctica (Fig. 2), where no member of the Asteraceae exists (Funk et al. 2005).
Records suggest that the genus Artemisia L. originated from Asia in the temperate, Arid and semi-arid regions during the Cenozoic era (about 66 mya). The centre of diversity was reported to be within the temperate regions of Eurasia and North America by Ling (1982). However, the greatest centre of diversity lies around the temperate areas of Asia with about 38% of the total species population. In Asia, the centre of origin for Artemisia L. was most probably in the mountain regions of north-western Asia, and diversification and development in the genus were possibly at a peak in the Cenozoic (Wang 2004). Artemisia species have a global widespread within the Asian continent leading in terms of diversity with about 82 of them endemic to China, 50 in Japan, about 35 in Iran (Table 2).
The genus is ecologically and economically significant with an age-long practice for different ethnobotanical usage such as medicinal herbs, source of food in different parts of the world, herbage for feeding livestock, and habitat (in steppe communities). The plant genus has a well-documented medicinal use with drugs like artemisinin, originally from A. annua, and presently isolated in the aerial part of about 12 species [such as A. bushriences], widely used as drugs and other pharmacological activities (Mannan et al. 2010; Martínez et al. 2012). They also possess notable economic status as aromatic and medicinal plants with ethnopharmacological properties owing to the different biological activities, including antimalarial, anti-inflammatory, immune-modulating and antioxidant activity (Khlifi et al. 2013; Kim et al. 2015; Woerdenbag et al. 1990).
Essential oils in Artemisia
Essential oils are compounds usually networked or multiplexed with volatile molecules such as terpenes and aromatic components that are phenol derivatives. They have a broad spectrum of bioactivity due to the presence of several active ingredients or secondary metabolites with varying modes of action, which make them play vital roles in nature, ranging from antibacterial, antiviral, antifungal, etc. (Dhifi et al. 2016). Artemisia species are an excellent source of essential oils such as pinene, thujyl alcohol, cadinene, phellandrene, thujone, etc. and have been reported to achieve remarkable success for several biological activities including, analgesic, anti-coccidial, anti-diabetic, antifungal, antiviral, anti-herpes virus, and lots more (Kumar and Kumari 2018; Martínez et al. 2012).
Anti-viral and immune-stimulatory potentials of Artemisia and Artemisia-based Products against SARS-CoV-2
Artemisia spp. had earlier been reported to consist of essential phytochemicals that contribute to its inhibitory role against viruses (Bora and Sharma 2010). Before the outbreak of COVID-19, some ethnopharmacological studies on Artemisia derivatives revolved around their retroviral properties (Efferth 2018; Jana et al. 2017; Laila et al. 2019; Lubbe et al. 2012), capacity to minimize the replication of herpes viruses (Efferth et al. 2008; Milbradt et al. 2009; Naesens et al. 2006; Nagamune et al. 2007) and inhibition of hepatitis B and C viruses (Dai et al. 2016; Paeshuyse et al. 2006; Qi et al. 2013; Romero et al. 2005), etc. Noteworthily, the bioactive constituents present in A. annua have demonstrated activity against several viruses such as bovine viral diarrhoea (Romero et al. 2006), Epstein-Barr Virus, and Hepatitis B Virus (Haq et al. 2020). Earlier, some authors reported the use of A. annua against SARS coronavirus which appeared in 2002 Lin et al. (2003). The presence of flavonoids, quercetin, and di-caffeoylquinic acid in the plant inhibits the activity of MERS-CoV-3 CLPro, an enzyme that is similarly produced by SARS-CoV-2 (Jo et al. 2019, 2020). Interestingly, in a Vero cell-based, 3-(4,5-dimethylthiazol-2-yl-)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay for virus-induced cytopathic effect (CPE) screening analysis of medicinal plant extracts with antiviral potentials against SAR-CoV viral strain (α-coronavirus), A. annua alongside three other plants demonstrated a substantial inhibitory effect (Li et al. 2005). The results showed that A. annua, a highly efficacious species demonstrated a CC50 of 1053.0(± 92.8) µg/ml and EC50 of 34.5 ± 2.6 µg/mL with a selective index > 31 as compared to interferon-α that was > 100,000(± 710.1) and 660.3(± 119.1) respectively, indicating its ability to inhibit SARS-CoV-2 penetration and replication.
Since its discovery as an antiviral agent by a Chinese scientist (Qian et al. 1982), several studies have revealed the promising role of Artemisinin and its derivatives in the inhibition of viruses (Efferth et al. 2008). Artemisinin has been revealed to inhibit replication and penetration of viruses both in vivo and in vitro as well as generating enhanced host type I interferon response (Wang et al. 2020a). The replication of Hepatitis C replicon, a single-stranded RNAvirus just like SARS-CoV-2 was reported to be inhibited by artemisinin (Obeid et al. 2013). Very recently, a study on molecular dynamic using computer-aided drug discovery (CADD) revealed that artemisinin and its derivatives could be more potent than hydroxychloroquine (HCQ) in silico. In addition to that, artemisinin and its derived molecules showed an extra mode of interaction by binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein and producing a better Vina docking score of − 7.1 kcal/mol for artenilic acid than − 5.5 kcal/mol for hydroxychloroquine (Sehailia and Chemat 2020). The study further revealed that the formed complexes interfered and remained stable on the SARS-CoV-2 Spike protein receptor site. Besides the antiviral activity, Artemisia contains a high concentration of zinc, which is reported to be effective for the immunomodulation effect of host response and increase in CD4 level (Honscheid et al. 2009). It should be noted that the antioxidant ability of Artemisia enhances immune defense.
Clinical interventional studies of Artemisia and Artemisia–based products as mono- or combined therapy in the face of COVID-19
From a safety point of view, hundreds of phytochemicals present in A. annua have been revealed to be below recommended toxicity limits (Duke 1992; Lutgen 2019; Yang et al. 2010). Some antiviral agents including repurposed off-label drugs such as CQ, HCQ, Redmesivir, etc. have been in the spotlight as frontline therapies for COVID-19 (Bolarin et al. 2020). However, some of them have demonstrated cardiotoxicity concerns among many other after-administration side-effects (Yang et al. 2010). Notably, Artemisinin has been reported to possess a better and lower toxicity profile compared with CQ and HCQ (Cheong et al. 2020). As such, clinicians can have minimal worries should higher dosage application become necessary. Also, its flexibility as a combination therapy with other drugs suggests its potential usage for the treatment of patients with cases of co-infections.
As of 19th of October 2020, only nine treatment intervention trials on Artemisia spp. and Artemisia products have been registered worldwide for COVID-19 (using Artemisinin, Artesunate, and COVID-19 as search notes) (ClinicalTrials.gov 2020). Six of these were registered in the United States National Library of Medicine while the other three appeared in the Chinese clinical trial registry (ChiCTR 2020), of which one is suspended (ChiCTR2000030082). Up to date, there exists only a randomized, double-blinded, placebo-controlled, parallel, multi-arm, multi-centre and phase II clinical trial (but not yet recruiting) on Artemisia annua herbal medicine (NCT04530617), which is hypothesized to give improved clinical outcomes in high-risk COVID-19 infected patients when introduced as an early intervention. The intervention of the study includes; tea 225 mg/1350 mg per day. Oral, one 8 oz brewed tea (two bags) three times a day, Day 1–14. The investigations are focused on evaluating the safety and efficacies on morbidity of COVID-19 patients (adults with mild symptoms) in decreasing the course of the disease and viral load in symptomatic stable positive swab COVID-19 patients (ClinicalTrials.gov 2020). More clinical interventional studies need to be conducted to further provide therapeutic protocols to substantiate the safety and efficacy of Artemisia and Artemisia products. The potential management of outpatients with COVID-19 and high-risk factors such as cardiovascular diseases needs to be ascertained using Artemisia and its products. Furthermore, clinical validation of the use of Artemisia either in mono-therapeutic form or as combination therapies with existing drugs, particularly with repurposed drugs of debatable safety profiles is vital. The registered clinical trial interventions using Artemisia annua and Artemisia-based products in mono or combination therapy for COVID-19 treatment are presented in Table 3.
Mechanistic action of Artemisia and Artemisia-based products on SARS-CoV-2
At present, the use of herbal medicine remains debatable worldwide; it is supposedly believed that they are associated with complications and adverse effects (Wang et al. 2020b). Their acceptability for COVID-19 management is based on the understanding of their mechanism of action (which is derived from experimental and predicted targets of their active chemical ingredients) and clinical profiles. These two important conditions are largely predicated on the knowledge and clinical profiling of the activities of the chemical ingredients isolated from the plant as described by Jiang et al. (2020). which are, therefore, useful for the following: One, molecular docking and target binding studies in target assessment (Huang et al. 2020). Two, multi-omics studies for finding clinically-relevant target (Wang et al. 2020b). Three, facilitating molecular and disease network analysis concerning the experimental and predicted targets for understanding the network pharmacology (Yang et al. 2020). Four, facilitating the statistical frequency of appearance analysis of literature-reported chemical ingredients and mechanisms for focusing on the high confidence mechanisms (Huang et al. 2020).
Generally, the mode of action of active ingredients from natural products against coronaviruses is through suppressing virus infection which in turn reduces the viral load (Jassim and Naji 2003). Specifically, the mode of action of A. annua on Spike protein of the SARS-CoV-2 is not clearly understood. Nevertheless, it has been reported to be by inhibiting the enzymatic activity of chymotrypsin-like protease (3CLpro) (Law et al. 2020). A. annua stimulates adaptive immunity by generating CD8 and CD4 lymphocytes responsible for the production of antibodies targeting SARS-CoV-2 and down-regulating the production of pro-inflammatory cytokines prostaglandin E2 (PGE2), TNF-α, interleukin-6 (IL-6), interleukin-10 (IL-10), thus increasing CD4 count and CD4/CD8 ratio (Poisson-Benatouil 2020). Cytokine storms decrease the number of Treg cell in COVID-19 infected patients, and leads to functionally exhausted CD8 and CD4 lymphocytes which ultimately affects human immune systems and cause severe respiratory failure (De Biasi et al. 2020).
Conclusion and perspective
The antiviral activity of Artemisia and its derivatives against SARS-CoV-2 have been extensively reviewed. Besides its antiviral activity, Artemisia is a super accumulator of zinc and has a well-known toxicological profile (Honscheid et al. 2009). Understanding the safety and efficacy when administered either as a monotherapy or combination therapy, mechanism of action, formulation and active dosage for COVID-19 drug development is required. We, therefore, recommend sufficient clinical interventional controlled-evidences to elucidate the most effective scheme of administration before integrating both Artemisia herbal medicine and Artemisia products into medicinal practice. Combined administration of Artemisia herbs or other Artemisia products with other antiviral or repurposed drugs should be conducted with stringent adherence to health protocols and clinical guidelines under the supervision of a medical practitioner to understand the drug-drug interaction and assess their effect on pro-inflammatory cytokines and hACE2 receptor.
Abbreviations
- hACE2:
-
Human Angiotensin-converting enzyme 2
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- COVID-19:
-
Coronavirus disease-2019
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- IL-2:
-
Interleukin-2
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- TNF-α:
-
Tumour necrosis factor-alpha
- CD8:
-
Cluster of differentiation antigen 8
- CD4:
-
Cluster of differentiation antigen 8
- MERS-CoV-3 CLPro:
-
Middle east respiratory syndrome-coronavirus-3 chymotrypsin-like protease
- EC50 :
-
Half-maximal effective concentration
- CC50 :
-
Half-cytotoxic concentration
- CADD:
-
Computer-aided drug discovery
- CQ:
-
Chloroquine
- HCQ:
-
Hydroxychloroquine
- 3CLpro :
-
Chymotrypsin-like protease
- PGE2:
-
Prostaglandin E2
References
Bolarin JA et al (2020) Therapeutic drugs for SARS-CoV-2 treatment: current state and perspective. Int Immunopharmacol 90:107228. https://doi.org/10.1016/j.intimp.2020.107228
Bora KS, Sharma A (2010) Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol 129:403–409
Boukhatem MN, Setzer WN (2020) Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: future perspectives. Plants 9:800. https://doi.org/10.3390/plants9060800
Brisibe EA et al (2009) Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem 115:1240–1246
Cheong DH, Tan WD, Wong WF, Tran T (2020) Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res. https://doi.org/10.1016/j.phrs.2020.104901
Chinese Clinical Trials Registry (2020) http://www.chictr.org.cn/searchprojen.aspx?title=&officialname=&subjectid=&secondaryid=&applier=&studyleader=ðicalcommitteesanction=&sponsor=&studyailment=COVID-19&studyailmentcode=&studytype=0&studystage=0&studydesign=0&minstudyexecutetime=&maxstudyexecutetime=&recruitmentstatus=0&gender=0&agreetosign=&secsponsor=®no=®status=0&country=&province=&city=&institution=&institutionlevel=&measure=arte&intercode=&sourceofspends=&createyear=0&isuploadrf=&whetherpublic=&btngo=btn&verifycode=&page=1. Accessed 20/10/2020
COVID19 Clinical Trials (2020) https://clinicaltrials.gov/. Accessed 15 August 2020
Dai R, Xiao X, Peng F, Li M, Gong G (2016) Artesunate, an anti-malarial drug, has a potential to inhibit HCV replication. Virus Genes 52:22–28
De Biasi S et al (2020) Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 11:1–17
Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W (2016) Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3:25
Duke JA (1992) Database of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, Boca Raton
Efferth T (2018) Beyond malaria: the inhibition of viruses by artemisinin-type compounds. Biotechnol Adv 36:1730–1737. https://doi.org/10.1016/j.biotechadv.2018.01.001
Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M (2008) The antiviral activities of artemisinin and artesunate. Clin Infect Dis 47:804–811
Ferreira JF (2007) Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J Agric Food Chem 55:1686–1694
Funk VA et al (2005) Everywhere but antarctica: using a supertree to understand the diversity and distribution of the compositae. Biol Skr 55:343–374
Ghafoor A (2002) Flora of Pakistan. asteraceae (1) Anthemideae vol 207. Missouri Botanical Garden, USA, Department of Botany, University of Karachi, Karachi-Pakistan
Haq FU et al. (2020) Artemisia annua: trials are needed for COVID‐19. Phytother Res
Honscheid A, Rink L, Haase H (2009) T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocr Metab Immune Disorders Drug Targets (Formerly Current Drug Targets-Immune, Endocrine and Metabolic Disorders) 9:132–144
Hu S-y (1965) The compositae of China Taiwan Museum. Q J 18:1–136
Huang F et al. (2020) A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19). Pharmacol Res 104929
IqbalHussain FAK, Khattak MUR (2011) Evaluation of inorganic profile of selected medicinal plants of Khyber Pakhtunkhwa Pakistan. World Appl Sci J 12:1464–1468
Jana S, Iram S, Thomas J, Hayat MQ, Pannecouque C, Dehaen W (2017) Application of the triazolization reaction to afford dihydroartemisinin derivatives with anti-HIV activity. Molecules 22:303
Jassim SAA, Naji MA (2003) Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 95:412–427
Jiang S, Cui Q, Ni B, Chen Y, Tan Y, Chen W, Chen YZ (2020) Databases for facilitating mechanistic investigations of traditional Chinese medicines against COVID-19. Pharmacol Res 159:104989
Jin Y-H et al (2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 7:4
Jo S, Kim H, Kim S, Shin DH, Kim MS (2019) Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Design 94:2023–2030
Jo S, Kim S, Shin DH, Kim M-S (2020) Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem 35:145–151
Khlifi D, Sghaier RM, Amouri S, Laouini D, Hamdi M, Bouajila J (2013) Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L and Peganum harmala L. Food Chem Toxicol 55:202–208
Kim W-S et al (2015) Anti-inflammatory, antioxidant and antimicrobial effects of Artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol 19:21
Kitamura S (1939) A classification of Artemisia. Acta Phytotax Geobot 8:62–66
Kitamura S (1940) Compositae Japonicae. Pars secunda Mem Coll Sci Kyoto Univ 15:258–446
Kumar S, Kumari R (2018) Artemisia: a medicinally Important Genus. J Complement Med Alt Healthcare 7:555723. https://doi.org/10.19080/JCMAH.2018.07.555723
Kurşat M, Cİvelek Ş, Türkoğlu İ, Tabur S, Gür N, (2015) A new species of subgenus Seriphidium of Artemisia L. (Asteraceae) from Turkey. Turk J Bot 39:88–95
Laila U et al (2019) Role of medicinal plants in HIV/AIDS therapy. Clin Exp Pharmacol Physiol 46:1063–1073. https://doi.org/10.1111/1440-1681.13151
Law S, Leung AW, Xu C (2020) Is the traditional Chinese herb “Artemisia annua” possible to fight against COVID-19? Integr Med Res 9
Li S-Y et al (2005) Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res 67:18–23
Lin L, Han Y, Yang Z (2003) Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western Medicine. Chin J Integr Trad Western Med 23:409–413
Ling Y (1982) On the system of the genus Artemisia L and the relationship with its allies. Bull Lab North East For Inst 2:1–60
Liu X, Cao J, Huang G, Zhao Q, Shen J (2019) Biological activities of artemisinin derivatives beyond malaria. Curr Top Med Chem 19:205–222. https://doi.org/10.2174/1568026619666190122144217
Lubbe A, Seibert I, Klimkait T, Van der Kooy F (2012) Ethnopharmacology in overdrive: the remarkable anti-HIV activity of Artemisia annua. J Ethnopharmacol 141:854–859
Lutgen P (2019) No toxicity detected for Artemisia annua or afra Review published online on August 5, 2019 on http://Malariaworld.org
Mannan A, Ahmed I, Arshad W, Asim MF, Qureshi RA, Hussain I, Mirza B (2010) Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar J 9:310. https://doi.org/10.1186/1475-2875-9-310
Martín J, Torrell M, Korobkov AA, Vallès J (2003) Palynological features as a systematic marker in Artemisia L. and related genera (Asteraceae, Anthemideae)-II: implications for Subtribe Artemisiinae delimitation. Plant Biol 5:85–93. https://doi.org/10.1055/s-2003-37979
Martínez MJA, Del Olmo LMB, Ticona LA, Benito PB (2012) The Artemisia L. genus: a review of bioactive sesquiterpene lactones. Studies in natural products chemistry, vol 37. Elsevier, Amstredam, pp 43–65
Milbradt J, Auerochs S, Korn K, Marschall M (2009) Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J Clin Virol 46:24–28
Naesens L, Bonnafous P, Agut H, De Clercq E (2006) Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J Clin Virol 37:S69–S75
Nagamune K, Moreno SN, Sibley LD (2007) Artemisinin-resistant mutants of Toxoplasma gondii have altered calcium homeostasis. Antimicrob Agents Chemother 51:3816–3823
Naghavi MR, Alaeimoghadam F, Ghafoori H (2014) Artemisia species from iran as valuable resources for medicinal uses. World Acad Sci Eng Technol 11:1058–1064
Nigam M et al (2019) Bioactive compounds and health benefits of artemisia species. Nat Prod Commun 14:1–17. https://doi.org/10.1177/1934578X19850354
No toxicity detected for Artemisia annua or afra (2019) www.malariaworld.org.
Obeid S et al (2013) Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS ONE 8:e81783
Paeshuyse J et al (2006) Hemin potentiates the anti-hepatitis C virus activity of the antimalarial drug artemisinin. Biochem Biophys Res Commun 348:139–144
Poisson-Benatouil C (2020) Action of Artemisia annua on adaptive immunity in COVID-19 infections. A concept note. pp 22. Available from: https://lavierebelle.org/action-del-artemisia-annua-sur-l?lang=en. Accessed 19 Sept 2020
Poljakov PP (1961) Systematic studies in the genus Artemisia L. Trudy Ins Bot Akad Nauk Kazakh SSR Alma Acta 11:134–177
Qi F et al (2013) Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discov Ther 7:212–224
Qian RS, Li Z, Yu J, Ma DJ (1982) The immunologic and antiviral effect of qinghaosu. J Trad Chin Med 2:271–276
Romero MR, Efferth T, Serrano MA, Castaño B, Macias RI, Briz O, Marin JJ (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiv Res 68:75–83
Romero MR, Serrano MA, Vallejo M, Efferth T, Alvarez M, Marin JJ (2006) Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med 72:1169–1174
Sehailia M, Chemat S (2020) Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1796809
Tong Y, Deng Z (2020) An aurora of natural products-based drug discovery is coming. Synth Syst Biotechnol 5:92–96. https://doi.org/10.1016/j.synbio.2020.05.003
Wang W-M (2004) On the origin and development of Artemisia (Asteraceae) in the geological past. Bot J Linn Soc 145:331–336. https://doi.org/10.1111/j.1095-8339.2004.00287.x
Wang X et al (2020a) Artemisinin inhibits the replication of flaviviruses by promoting the type I interferon production. Antivir Res 179:104810
Wang Y, Zeng X, Zhao Y, Chen W, Chen YZ (2020b) The pros and cons of traditional Chinese medicines in the treatment of COVID-19. Pharmacol Res 157:104873. https://doi.org/10.1016/j.phrs.2020.104873
WHO (2015) Guidelines for the treatment of malaria, 3rd edn. World Health Organization, Geneva
WHO (2020a) Coronavirus disease 2019 (COVID-19): situation report, 72. In, 2020a. World Health Organization, Geneva
WHO (2020b) WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. https://covid19.who.int/
WHO Director-General’s Remark at the media briefing on 2019 n-CoV on 11 February 2020 (2020c). https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed 31 March 2021.
Woerdenbag HJ, Lugt CB, Pras N (1990) Artemisia annua L.: a source of novel antimalarial drugs. Pharm Weekbl 12:169–181
Wright CW (2001) Artemisia. Medicinal and Aromatic Plant—industrial profile. CRC Press, London
Yang B, Zhou S, Li C, Wang Y (2010) Toxicity and side effects of artemisiae annuae CQ-189. J Chin Mater Med 35:204–207
Yang R et al (2020) Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res 157:104820
Yu H, Zhong S (2001) Artemisia species in traditional Chinese medicine and the discovery of artemisinin. CRC Press, Boca Raton
Zyad A, Tilaoui M, Jaafari A, Oukerrou MA, Mouse HA (2018) More insights into the pharmacological effects of artemisinin. Phytother Res 32:216–229. https://doi.org/10.1002/ptr.5958
Acknowledgements
We acknowledge the painstaking efforts of all reviewers toward the enhanced improvement of this work.
Author information
Authors and Affiliations
Contributions
Joshua Iseoluwa Orege conceptualized and designed the research idea and was involved in the review of the literature with Sherif Babatunde Adeyemi, Bashir Bolaji Tiamiyu, Toluwanimi Oluwadara Akinyemi, Yusuf Ajibola Ibrahim, Odunola Blessing Orege. All authors contributed to the drafting of the manuscript and the final review of the final draft of the manuscript. All authors contributed to the final version and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies involving animals performed by any of the authors. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
Joshua Iseoluwa Orege, Sherif Babatunde Adeyemi, Bashir Bolaji Tiamiyu,Toluwanimi Oluwadara Akinyemi, Yusuf Ajibola Ibrahim, Odunola Blessing Orege declare that they have no conclict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orege, J.I., Adeyemi, S.B., Tiamiyu, B.B. et al. Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective. ADV TRADIT MED (ADTM) 23, 85–96 (2023). https://doi.org/10.1007/s13596-021-00576-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-021-00576-5