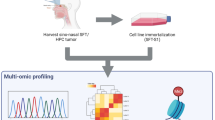
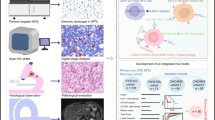
Abstract
Solitary fibrous tumors (SFTs) are rare mesenchymal tumors characterized by recurrent NAB2::STAT6 gene fusion, which are associated with an unpredictable clinical course, including the potential for recurrence or metastasis. Current therapeutic approaches for relapsed cases remain ineffective, and there is no established standard of care for SFTs. Although patient-derived cancer cell lines are fundamental research tools, only a few cell lines have been developed for SFTs. To address this, we established a novel SFT cell line, NCC-SFT1-C1, derived from surgically resected tumor tissues of a patient with SFT. The cell line retains the characteristic recurrent NAB2::STAT6 gene fusion in concordance with the matched original tumor. These cells exhibit moderate proliferation, invasion ability, and spheroid formation. We demonstrated that NCC-SFT1-C1 cells are useful for the high-throughput screening of the antiproliferative effects of 221 oncology drugs. Therefore, the NCC-SFT1-C1 cell line is a valuable tool for basic and preclinical studies on SFT.




Similar content being viewed by others
References
Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–5.
Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–2.
Mohajeri A, Tayebwa J, Collin A, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013;52:873–86.
Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48:63–74.
Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009;21:327–31.
Salas S, Resseguier N, Blay JY, et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann Oncol. 2017;28:1979–87.
van Houdt WJ, Westerveld CM, Vrijenhoek JE, et al. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol. 2013;20:4090–5.
de Bernardi A, Dufresne A, Mishellany F, Blay JY, Ray-Coquard I, Brahmi M. Novel therapeutic options for solitary fibrous tumor: antiangiogenic therapy and beyond. Cancers. 2022;14:1064.
Ben-David U, Beroukhim R, Golub TR. Genomic evolution of cancer models: perils and opportunities. Nat Rev Cancer. 2019;19:97–109.
Saito S, Morita K, Kohara A, et al. Use of BAC array CGH for evaluation of chromosomal stability of clinically used human mesenchymal stem cells and of cancer cell lines. Hum Cell. 2011;24:2–8.
Ben-David U, Siranosian B, Ha G, et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature. 2018;560:325–30.
Yang W, Soares J, Greninger P, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–61.
Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7.
Goodspeed A, Heiser LM, Gray JW, Costello JC. Tumor-derived cell lines as molecular models of cancer pharmacogenomics. Mol Cancer Res. 2016;14:3–13.
Hattori E, Oyama R, Kondo T. Systematic review of the current status of human sarcoma cell lines. Cells. 2019;8:157.
Lee JY, Guan P, Lim AH, et al. Establishment and characterization of a patient-derived solitary fibrous tumor/hemangiopericytoma cell line model. Hum Cell. 2024;37:310–22.
Ghanim B, Baier D, Pirker C, et al. Trabectedin is active against two novel, patient-derived solitary fibrous pleural tumor cell lines and synergizes with ponatinib. Cancers. 2022;14:5602.
Yoshimatsu Y, Noguchi R, Tsuchiya R, et al. Establishment and characterization of NCC-ASPS1-C1: a novel patient-derived cell line of alveolar soft-part sarcoma. Hum Cell. 2020;33:1302.
Bairoch A. The cellosaurus, a cell-Line knowledge resource. J Biomol Tech. 2018;29:25–38.
Klemperer P, Rabin CB. Primary neoplasms of the pleura. A report of five cases. Arch Pathol. 1931;11:385–412.
Barthelmeß S, Geddert H, Boltze C, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184:1209–18.
Bieg M, Moskalev EA, Will R, et al. Gene expression in solitary fibrous tumors (SFTs) correlates with anatomic localization and NAB2-STAT6 gene fusion variants. Am J Pathol. 2021;191:602–17.
Ishihara S, Ogura K, Maejima A, et al. Predictive value of peripheral blood markers in soft tissue sarcoma patients treated with eribulin. Jpn J Clin Oncol. 2023;53:494–500.
Kawai A, Narahara H, Takahashi S, et al. Safety and effectiveness of eribulin in Japanese patients with soft tissue sarcoma including rare subtypes: a post-marketing observational study. BMC Cancer. 2022;22:528.
Kawai A, Yonemori K, Takahashi S, Araki N, Ueda T. Systemic therapy for soft tissue sarcoma: proposals for the optimal use of pazopanib, trabectedin, and eribulin. Adv Ther. 2017;34:1556–71.
Kawai A, Araki N, Naito Y, et al. Phase 2 study of eribulin in patients with previously treated advanced or metastatic soft tissue sarcoma. Jpn J Clin Oncol. 2017;47:137–44.
Logan TF. Foretinib (XL880): c-MET inhibitor with activity in papillary renal cell cancer. Curr Oncol Rep. 2013;15:83–90.
Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31:181–6.
Shah MA, Wainberg ZA, Catenacci DV, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS ONE. 2013;8: e54014.
Knubel KH, Pernu BM, Sufit A, Nelson S, Pierce AM, Keating AK. MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget. 2014;5:1338–51.
Goździk-Spychalska J, Szyszka-Barth K, Spychalski L, et al. C-MET inhibitors in the treatment of lung cancer. Curr Treat Options Oncol. 2014;15:670–82.
Rayson D, Lupichuk S, Potvin K, et al. Canadian cancer trials group IND197: a phase II study of foretinib in patients with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2-negative recurrent or metastatic breast cancer. Breast Cancer Res Treat. 2016;157:109–16.
Yau TCC, Lencioni R, Sukeepaisarnjaroen W, et al. A phase I/II multicenter study of single-agent foretinib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2017;23:2405–13.
Seiwert T, Sarantopoulos J, Kallender H, McCallum S, Keer HN, Blumenschein G Jr. Phase II trial of single-agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31:417–24.
Konstantinopoulos PA, Vandoros GP, Papavassiliou AG. FK228 (depsipeptide): a HDAC inhibitor with pleiotropic antitumor activities. Cancer Chemother Pharmacol. 2006;58:711–5.
Shimony S, Horowitz N, Ribakovsky E, et al. Romidepsin treatment for relapsed or refractory peripheral and cutaneous T-cell lymphoma: real-life data from a national multicenter observational study. Hematol Oncol. 2019;37:569–77.
De Luca A, Normanno N. Tivozanib, a pan-VEGFR tyrosine kinase inhibitor for the potential treatment of solid tumors. IDrugs : The Investigational Drugs Journal. 2010;13:636–45.
Beckermann KE, Asnis-Alibozek AG, Atkins MB, et al. Long-term survival in patients with relapsed/refractory advanced renal cell carcinoma treated with tivozanib: analysis of the phase III TIVO-3 trial. Oncologist. 2024;29:254–62.
Pratesi G, Casazza AM, Di Marco A. Antitumor activity of N-trifuloroacetyladriamycin-14-valerate. Cancer Treat Rep. 1978;62:105–10.
Sharma P, Zargar-Shoshtari K, Sexton WJ. Valrubicin in refractory non-muscle invasive bladder cancer. Expert Rev Anticancer Ther. 2015;15:1379–87.
Acknowledgements
We thank Drs. S. Iwata, K. Ogura, and H. Kondo (Department of Musculoskeletal Oncology and Rehabilitation Medicine, the National Cancer Center Hospital) for sampling tumor tissue specimens from surgically resected materials. We also appreciate the technical assistance provided by Mrs. Y. Shiotani (Central Animal Division, National Cancer Center Research Institute). We would also like to thank Editage (www.editage.jp) for their help with English language editing and their constructive comments on the manuscript. This research was technically assisted by the Fundamental Innovative Oncology Core at the National Cancer Center.
Funding
This research was supported by the Japan Agency for Medical Research and Development (grant number: 20ck0106537h).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the National Cancer Center (approval number 2004–050).
Informed consent
Written informed consent for publication was provided by the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwata, S., Noguchi, R., Oosaki, J. et al. Establishment and characterization of NCC-SFT1-C1: a novel patient-derived cell line of solitary fibrous tumor. Human Cell 38, 49 (2025). https://doi.org/10.1007/s13577-025-01175-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13577-025-01175-1