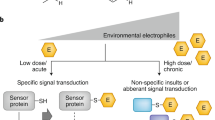
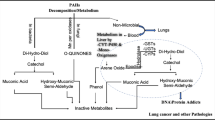
Abstract
The effect of air pollution on public health is severely detrimental. In humans; the physiological response against pollutants is mainly elicited via the activation of aryl hydrocarbon receptor (AhR). It acts as a prime sensor of xenobiotic chemicals, also functioning as a transcription factor regulating a variety of gene expressions. Along with AhR, another pivotal element of the pollution stress pathway is Xenobiotic Response Elements (XREs). XRE, as studied are some conserved sequences in the DNA, responsible for the physiological response against pollutants. XRE is present at the upstream of the inducible target genes of AhR and it regulates the function of the AhR. XRE(s) are highly conserved in species as it has only eight specific sequences found so far in humans, mice, and rats. Inhalation of toxicants like dioxins, gaseous industrial effluents, and smoke from burning fuel and tobacco leads to predominant damage to the lungs. However, scientists are exploring the involvement of AhR in chronic diseases for example chronic obstructive pulmonary disease (COPD) and also other lethal diseases like lung cancer. In this review, we summarise what is known at this time about the roles played by the XRE and AhR in our molecular systems that have a defined control in the normal maintenance of homeostasis as well as dysfunctions.
Graphical abstract







Similar content being viewed by others
Data availability
All data, which are included in the article are available within the paper.
References
Li S, Pei X, Zhang W, Xie HQ, Zhao B. Functional analysis of the dioxin response elements (DREs) of the murine CYP1A1 gene promoter: beyond the core DRE sequence. Int J Mol Sci. 2014;15(4):6475–87. https://doi.org/10.3390/ijms15046475.
Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357(1):155–63. https://doi.org/10.1006/abbi.1998.0814.
Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141(1–2):3–24. https://doi.org/10.1016/s0009-2797(02)00063-7.
Ma Q, Whitlock JP. A Novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin*. J Biol Chem. 1997;272(14):8878–84. https://doi.org/10.1074/jbc.272.14.8878.
Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18(2):978–88. https://doi.org/10.1128/MCB.18.2.978.
Hord NG, Perdew GH. Physicochemical and immunocytochemical analysis of the aryl hydrocarbon receptor nuclear translocator: characterization of two monoclonal antibodies to the aryl hydrocarbon receptor nuclear translocator. Mol Pharmacol. 1994;46(4):618–26.
Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45(3):428–38.
Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;44(3):511–8.
Bank PA, Yao EF, Phelps CL, Harper PA, Denison MS. Species-specific binding of transformed Ah receptor to a dioxin responsive transcriptional enhancer. Eur J Pharmacol. 1992;228(2–3):85–94. https://doi.org/10.1016/0926-6917(92)90016-6.
Ma Q. Induction of CYP1A1. The AhR/DRE paradigm: transcription, receptor regulation, and expanding biological roles. Curr Drug Metab. 2001;2(2):149–64. https://doi.org/10.2174/1389200013338603.
Shen ES, Whitlock JP Jr. The potential role of DNA methylation in the response to 2,3,7,8-tetrachlorodibenzo-<em>p</em>-dioxin *. J Biol Chem. 1989;264(30):17754–8. https://doi.org/10.1016/S0021-9258(19)84636-7.
Henry EC, Rucci G, Gasiewicz TA. Characterization of multiple forms of the Ah receptor: comparison of species and tissues. Biochemistry. 1989;28(15):6430–40. https://doi.org/10.1021/bi00441a041.
Jones KW, Whitlock JPJ. Functional analysis of the transcriptional promoter for the CYP1A1 gene. Mol Cell Biol. 1990;10(10):5098–105. https://doi.org/10.1128/mcb.10.10.5098-5105.1990.
Jones PB, Durrin LK, Fisher JM, Whitlock JPJ. Control of gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Multiple dioxin-responsive domains 5’-ward of the cytochrome P1–450 gene. J Biol Chem. 1986;261(15):6647–50.
Jones PB, Durrin LK, Galeazzi DR, Whitlock JPJ. Control of cytochrome P1–450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci USA. 1986;83(9):2802–6. https://doi.org/10.1073/pnas.83.9.2802.
Denison MS, Fisher JM, Whitlock JPJ. Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci USA. 1988;85(8):2528–32. https://doi.org/10.1073/pnas.85.8.2528.
Fisher JM, Wu L, Denison MS, Whitlock JPJ. Organization and function of a dioxin-responsive enhancer. J Biol Chem. 1990;265(17):9676–81.
Kolluri SK, Jin U-H, Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol. 2017;91(7):2497–513. https://doi.org/10.1007/s00204-017-1981-2.
“Tissue expression of AHR - Summary - The Human Protein Atlas.” https://www.proteinatlas.org/ENSG00000106546-AHR/tissue. Accessed 12 Jan 2023
Chiba T, Uchi H, Yasukawa F, Furue M. Role of the arylhydrocarbon receptor in lung disease. Int Arch Allergy Immunol. 2011;155(Suppl):129–34. https://doi.org/10.1159/000327499.
Kawajiri K, Fujii-Kuriyama Y. The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Exp Anim. 2017;66(2):75–89. https://doi.org/10.1538/expanim.16-0092.
Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–7. https://doi.org/10.1074/jbc.270.3.1230.
Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. https://doi.org/10.1146/annurev.pa.22.040182.002505.
Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191(2):297–305. https://doi.org/10.1006/dbio.1997.8758.
Haarmann-Stemmann T, Abel J. The arylhydrocarbon receptor repressor (AhRR): structure, expression, and function. Biol Chem. 2006;387(9):1195–9. https://doi.org/10.1515/BC.2006.147.
Chiba T, Uchi H, Tsuji G, Gondo H, Moroi Y, Furue M. Arylhydrocarbon receptor (AhR) activation in airway epithelial cells induces MUC5AC via reactive oxygen species (ROS) production. Pulm Pharmacol Ther. 2011;24(1):133–40. https://doi.org/10.1016/j.pupt.2010.08.002.
Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. https://doi.org/10.1038/nature06880.
Fujisawa-Sehara A, Sogawa K, Nishi C, Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986;14(3):1465–77. https://doi.org/10.1093/nar/14.3.1465.
Denison MS, Fisher JM, Whitlock JPJ. The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988;263(33):17221–4.
Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265(24):14648–53.
Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Characterization of a DNA-protein interaction at the antioxidant responsive element and induction by 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1993;268(26):19875–81.
Fujisawa-Sehara A, Yamane M, Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci USA. 1988;85(16):5859–63. https://doi.org/10.1073/pnas.85.16.5859.
Yamamoto K, Nakano M, Ishihama A. Regulatory role of transcription factor SutR (YdcN) in sulfur utilization in Escherichia coli. Microbiology. 2015;161(Pt 1):99–111. https://doi.org/10.1099/mic.0.083550-0.
Julian T, Michel R, Victor S, Ina A, Sylvie E. Transcription inhibitors with XRE DNA-binding and cupin signal-sensing domains drive metabolic diversification in pseudomonas. mSystems. 2021;6(1):e00753-e820. https://doi.org/10.1128/mSystems.00753-20.
Kiely PD, J. O'Callaghan, Abbas A, F. O'Gara. Genetic analysis of genes involved in dipeptide metabolism and cytotoxicity in Pseudomonas aeruginosa PAO1. Microbiology. 2008;154(8):2209–18. https://doi.org/10.1099/mic.0.2007/015032-0.
Jackson DP, Joshi AD, Elferink CJ. Ah receptor pathway intricacies; signaling through diverse protein partners and DNA-motifs. Toxicol Res (Camb). 2015;4(5):1143–58. https://doi.org/10.1039/c4tx00236a.
Panteleyev AA, Bickers DR. Dioxin-induced chloracne—reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp Dermatol. 2006;15(9):705–30. https://doi.org/10.1111/J.1600-0625.2006.00476.X.
Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340(Pt 3):715–22.
Beischlag TV, et al. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol Cell Biol. 2002;22(12):4319–33. https://doi.org/10.1128/MCB.22.12.4319-4333.2002.
Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem. 1999;274(40):28708–15. https://doi.org/10.1074/jbc.274.40.28708.
Xing X, et al. SUMOylation of AhR modulates its activity and stability through inhibiting its ubiquitination. J Cell Physiol. 2012;227(12):3812–9. https://doi.org/10.1002/jcp.24092.
Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50(10):897–907. https://doi.org/10.1002/mnfr.200600006.
Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31(8):1319–28. https://doi.org/10.1093/carcin/bgq028.
Shimizu Y, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97(2):779–82. https://doi.org/10.1073/pnas.97.2.779.
Shi S, et al. The aryl hydrocarbon receptor nuclear translocator (Arnt) is required for tumor initiation by benzo[a]pyrene. Carcinogenesis. 2009;30(11):1957–61. https://doi.org/10.1093/CARCIN/BGP201.
Thum T, Erpenbeck VJ, Moeller J, Hohlfeld JM, Krug N, Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environ Health Perspect. 2006;114(11):1655–61. https://doi.org/10.1289/ehp.8861.
Kim JH, Sherman ME, Curriero FC, Guengerich FP, Strickland PT, Sutter TR. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol Appl Pharmacol. 2004;199(3):210–9. https://doi.org/10.1016/j.taap.2003.11.015.
Matsumoto Y, et al. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ Sci Technol. 2009;41(10):3775–80. https://doi.org/10.1021/ES062793G.
McLemore TL, et al. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990;82(16):1333–9. https://doi.org/10.1093/jnci/82.16.1333.
Oyama T, et al. Cytochrome P450 expression (CYP) in non-small cell lung cancer. Front Biosci. 2007;12:2299–308. https://doi.org/10.2741/2232.
Lin P, Chang H, Ho WL, Wu M-H, Su J-M. Association of aryl hydrocarbon receptor and cytochrome P4501B1 expressions in human non-small cell lung cancers. Lung Cancer. 2003;42(3):255–61. https://doi.org/10.1016/s0169-5002(03)00359-3.
Nerurkar PV, et al. Ahr locus phenotype in congenic mice influences hepatic and pulmonary DNA adduct levels of 2-amino-3-methylimidazo[4,5-f]quinoline in the absence of cytochrome P450 induction. Mol Pharmacol. 1996;49(5):874–81.
Nerurkar PV, et al. DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) in colon, bladder, and kidney of congenic mice differing in Ah responsiveness and N-acetyltransferase genotype. Cancer Res. 1995;55(14):3043–9.
Revel A, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23(4):255–61. https://doi.org/10.1002/jat.916.
Sagredo C, Øvrebø S, Haugen A, Fujii-Kuriyama Y, Baera R, Botnen IV, Mollerup S. Quantitative analysis of benzo[a]pyrene biotransformation and adduct formation in Ahr knockout mice. Toxicol Lett. 2006;167(3):173–82. https://doi.org/10.1016/j.toxlet.2006.09.005. (Epub 2006 Oct 16 PMID: 17049425).
Wong K-K, Jacks T, Dranoff G. NF-kappaB fans the flames of lung carcinogenesis. Cancer Prev Res (Phila). 2010;3(4):403–5. https://doi.org/10.1158/1940-6207.CAPR-10-0042.
Chen P-H, Chang H, Chang JT, Lin P. Aryl hydrocarbon receptor in association with RelA modulates IL-6 expression in non-smoking lung cancer. Oncogene. 2012;31(20):2555–65. https://doi.org/10.1038/onc.2011.438.
Dalwadi H, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(21):7674–82. https://doi.org/10.1158/1078-0432.CCR-05-1205.
Diry M, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25(40):5570–4. https://doi.org/10.1038/sj.onc.1209553.
Rico de Souza A, Zago M, Pollock SJ, Sime PJ, Phipps RP, Baglole CJ. Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis. J Biol Chem. 2011;286(50):43214–28. https://doi.org/10.1074/jbc.M111.258764.
Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386(6623):403–7. https://doi.org/10.1038/386403a0.
Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem. 2009;284(37):25135–48. https://doi.org/10.1074/jbc.M109.013292.
Wang JH, Trigg CJ, Devalia JL, Jordan S, Davies RJ. Effect of inhaled beclomethasone dipropionate on expression of proinflammatory cytokines and activated eosinophils in the bronchial epithelium of patients with mild asthma. J Allergy Clin Immunol. 1994;94(6 Pt 1):1025–34. https://doi.org/10.1016/0091-6749(94)90121-x.
Wong PS, Vogel CF, Kokosinski K, Matsumura F. Arylhydrocarbon receptor activation in NCI-H441 cells and C57BL/6 mice: possible mechanisms for lung dysfunction. Am J Respir Cell Mol Biol. 2010;42(2):210–7. https://doi.org/10.1165/rcmb.2008-0228OC.
White KE, et al. Prostaglandin E2 mediates IL-1β-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors1. J Immunol. 2008;180(1):637–46. https://doi.org/10.4049/jimmunol.180.1.637.
Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol. 2010;245(1):91–9. https://doi.org/10.1016/j.taap.2010.02.007.
Murray JJ, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315(13):800–4. https://doi.org/10.1056/NEJM198609253151304.
Chiba T, et al. Possible novel receptor for PGD2 on human bronchial epithelial cells. Int Arch Allergy Immunol. 2007;143(Suppl. 1):23–7. https://doi.org/10.1159/000101400.
Seltzer J, et al. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986;60(4):1321–6. https://doi.org/10.1152/jappl.1986.60.4.1321.
Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1304–9. https://doi.org/10.1164/AJRCCM.163.6.2009116. PMID: 11371392.
Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111(2):476–94. https://doi.org/10.1016/j.pharmthera.2005.10.015.
Tsuji G, et al. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J Dermatol Sci. 2011;62(1):42–9. https://doi.org/10.1016/j.jdermsci.2010.10.017.
Martinez JM, Afshari CA, Bushel PR, Masuda A, Takahashi T, Walker NJ. Differential toxicogenomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and nonmalignant human airway epithelial cells. Toxicol Sci. 2002;69(2):409–23. https://doi.org/10.1093/toxsci/69.2.409.
Standiford T, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest. 1991;86:1945–53. https://doi.org/10.1172/JCI114928.
Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999;160(3):1035–42. https://doi.org/10.1164/AJRCCM.160.3.9902064.
Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007;56(4):341–8. https://doi.org/10.2332/allergolint.R-07-153.
Guo J, et al. Expression of genes in the TGF-beta signaling pathway is significantly deregulated in smooth muscle cells from aorta of aryl hydrocarbon receptor knockout mice. Toxicol Appl Pharmacol. 2004;194:79–89. https://doi.org/10.1016/j.taap.2003.09.002.
Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L391–9. https://doi.org/10.1152/ajplung.00062.2005.
Profita M, et al. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Cell Mol Physiol. 2010;298(2):L261–9. https://doi.org/10.1152/ajplung.90593.2008.
Beamer CA, Shepherd DM. Role of the aryl hydrocarbon receptor (AhR) in lung inflammation. Semin Immunopathol. 2013;35(6):693–704. https://doi.org/10.1007/s00281-013-0391-7.
Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol. 2009;77(4):746–60. https://doi.org/10.1016/j.bcp.2008.11.021.
Chiba T, Chihara J, Furue M. Role of the arylhydrocarbon receptor (AhR) in the pathology of asthma and COPD. J Allergy. 2012;2012:372384. https://doi.org/10.1155/2012/372384.
Hanlon PR, Ganem LG, Cho YC, Yamamoto M, Jefcoate CR. AhR- and ERK-dependent pathways function synergistically to mediate 2,3,7,8-tetrachlorodibenzo-p-dioxin suppression of peroxisome proliferator-activated receptor-gamma1 expression and subsequent adipocyte differentiation. Toxicol Appl Pharmacol. 2003;189(1):11–27. https://doi.org/10.1016/s0041-008x(03)00083-8.
Cho YC, Zheng W, Yamamoto M, Liu X, Hanlon PR, Jefcoate CR. Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of Ah Receptor. Arch Biochem Biophys. 2005;439(2):139–53. https://doi.org/10.1016/j.abb.2005.04.025.
Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121(13):2402–14. https://doi.org/10.1182/blood-2012-09-378653.
Tauchi M, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25(21):9360–8. https://doi.org/10.1128/MCB.25.21.9360-9368.2005.
Marcus RS, Holsapple MP, Kaminski NE. Lipopolysaccharide activation of murine splenocytes and splenic B cells increased the expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator. J Pharmacol Exp Ther. 1998;287(3):1113–8.
Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J Allergy Clin Immunol. 1995;95(1 Pt 1):103–15. https://doi.org/10.1016/s0091-6749(95)70158-3.
Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int. 2015;2015:327470. https://doi.org/10.1155/2015/327470.
Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64(2):477–85. https://doi.org/10.1016/j.cyto.2013.07.022.
Ramirez JM, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40(9):2450–9. https://doi.org/10.1002/eji.201040461.
Ishihara Y, Kado SY, Hoeper C, Harel S, Vogel CFA. Role of NF-kB RelB in aryl hydrocarbon receptor-mediated ligand specific effects. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20112652.
Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721–6. https://doi.org/10.1073/pnas.0804231105.
Vogel CFA, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21(12):2941–55. https://doi.org/10.1210/me.2007-0211.
Busbee PB, et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.127551.
Beamer CA, Kreitinger JM, Cole SL, Shepherd DM. Targeted deletion of the aryl hydrocarbon receptor in dendritic cells prevents thymic atrophy in response to dioxin. Arch Toxicol. 2019;93(2):355–68. https://doi.org/10.1007/s00204-018-2366-x.
Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175(1):90–103. https://doi.org/10.4049/jimmunol.175.1.90.
Fullerton AM, Roth RA, Ganey PE. 2,3,7,8-TCDD enhances the sensitivity of mice to concanavalin A immune-mediated liver injury. Toxicol Appl Pharmacol. 2013;266(2):317–27. https://doi.org/10.1016/j.taap.2012.11.009.
Neamah WH, et al. AhR activation leads to massive mobilization of myeloid-derived suppressor cells with immunosuppressive activity through regulation of CXCR2 and MicroRNA miR-150-5p and miR-543-3p that target anti-inflammatory genes. J Immunol. 2019;203(7):1830–44. https://doi.org/10.4049/jimmunol.1900291.
Neamah WH, Busbee PB, Alghetaa H, Abdulla OA, Nagarkatti M, Nagarkatti P. AhR activation leads to alterations in the gut microbiome with consequent effect on induction of myeloid derived suppressor cells in a CXCR2-dependent manner. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21249613.
Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6(8):e23522. https://doi.org/10.1371/journal.pone.0023522.
Li S, et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity. 2018;49(5):915-928.e5. https://doi.org/10.1016/j.immuni.2018.09.015.
Lund AK, Goens MB, Nuñez BA, Walker MK. Characterizing the role of endothelin-1 in the progression of cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice. Toxicol Appl Pharmacol. 2006;212(2):127–35. https://doi.org/10.1016/j.taap.2005.07.005.
Ichihara S, et al. Ablation of aryl hydrocarbon receptor promotes angiotensin II-induced cardiac fibrosis through enhanced c-Jun/HIF-1α signaling. Arch Toxicol. 2019;93(6):1543–53. https://doi.org/10.1007/s00204-019-02446-1.
Andreola F, Fernandez-Salguero PM, Chiantore MV, Petkovich MP, Gonzalez FJ, De Luca LM. Aryl hydrocarbon receptor knockout mice (AHR-/-) exhibit liver retinoid accumulation and reduced retinoic acid metabolism. Cancer Res. 1997;57(14):2835–8.
Andreola F, et al. Mouse liver CYP2C39 is a novel retinoic acid 4-hydroxylase. Its down-regulation offers a molecular basis for liver retinoid accumulation and fibrosis in aryl hydrocarbon receptor-null mice. J Biol Chem. 2004;279(5):3434–8. https://doi.org/10.1074/jbc.M305832200.
Andreola F, et al. Reversal of liver fibrosis in aryl hydrocarbon receptor null mice by dietary vitamin A depletion. Hepatology. 2004;39(1):157–66. https://doi.org/10.1002/hep.20004.
Corchero J, Martín-Partido G, Dallas SL, Fernández-Salguero PM. Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-beta-binding protein-1. Int J Exp Pathol. 2004;85(5):295–302. https://doi.org/10.1111/j.0959-9673.2004.00397.x.
Tian J, Feng Y, Fu H, Xie HQ, Jiang JX, Zhao B. The aryl hydrocarbon receptor: a key bridging molecule of external and internal chemical signals. Environ Sci Technol. 2015;49(16):9518–31. https://doi.org/10.1021/acs.est.5b00385.
Lamb CL, et al. Aryl hydrocarbon receptor activation by TCDD modulates expression of extracellular matrix remodeling genes during experimental liver fibrosis. Biomed Res Int. 2016;2016:5309328. https://doi.org/10.1155/2016/5309328.
Mohammadi-Bardbori A, Bastan F, Akbarizadeh A-R. The highly bioactive molecule and signal substance 6-formylindolo[3,2-b]carbazole (FICZ) plays bi-functional roles in cell growth and apoptosis in vitro. Arch Toxicol. 2017;91(10):3365–72. https://doi.org/10.1007/s00204-017-1950-9.
Farmahin R, Crump D, O’Brien JM, Jones SP, Kennedy SW. Time-dependent transcriptomic and biochemical responses of 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are explained by AHR activation time. Biochem Pharmacol. 2016;115:134–43. https://doi.org/10.1016/j.bcp.2016.06.005.
Smith BW, Stanford EA, Sherr DH, Murphy GJ. Genome editing of the CYP1A1 locus in iPSCs as a platform to map AHR expression throughout human development. Stem Cells Int. 2016;2016:2574152. https://doi.org/10.1155/2016/2574152.
Wincent E, Kubota A, Timme-Laragy A, Jönsson ME, Hahn ME, Stegeman JJ. Biological effects of 6-formylindolo[3,2-b]carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem Pharmacol. 2016;110–111:117–29. https://doi.org/10.1016/j.bcp.2016.04.012.
Sibilano R, et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol. 2012;189(1):120–7. https://doi.org/10.4049/jimmunol.1200009.
Xie G, Peng Z, Raufman J-P. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G1006–15. https://doi.org/10.1152/ajpgi.00427.2011.
Liu M, et al. Emerging biological functions of IL-17A: a new target in chronic obstructive pulmonary disease? Front Pharmacol. 2021;12:695957. https://doi.org/10.3389/fphar.2021.695957.
Hattori N, et al. Compounds in cigarette smoke induce EGR1 expression via the AHR, resulting in apoptosis and COPD. J Biochem. 2022;172(6):365–76. https://doi.org/10.1093/jb/mvac077.
Pollet M, et al. The AHR represses nucleotide excision repair and apoptosis and contributes to UV-induced skin carcinogenesis. Cell Death Differ. 2018;25(10):1823–36. https://doi.org/10.1038/s41418-018-0160-1.
Bekki K, et al. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13. https://doi.org/10.1016/j.pestbp.2014.12.021.
Zhang X, et al. The aryl hydrocarbon receptor ligand ITE inhibits cell proliferation and migration and enhances sensitivity to drug-resistance in hepatocellular carcinoma. J Cell Physiol. 2021;236(1):178–92. https://doi.org/10.1002/jcp.29832.
Vogel CFA, et al. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171(5):1538–48. https://doi.org/10.2353/ajpath.2007.070406.
Raggi F, et al. Divergent effects of dioxin- or non-dioxin-like polychlorinated biphenyls on the apoptosis of primary cell culture from the mouse pituitary gland. PLoS One. 2016;11(1):e0146729. https://doi.org/10.1371/journal.pone.0146729.
Acknowledgements
Our research is supported by the University Grants Commission (Govt. of India), the Department of Biotechnology (Govt. of India), the Science and Engineering Research Board (Govt. of India), and the Department of Science and Technology (Govt. of India).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose. All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mandal, A., Biswas, N. & Alam, M.N. Implications of xenobiotic-response element(s) and aryl hydrocarbon receptor in health and diseases. Human Cell 36, 1638–1655 (2023). https://doi.org/10.1007/s13577-023-00931-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00931-5