Abstract
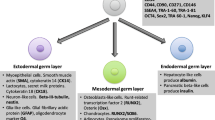
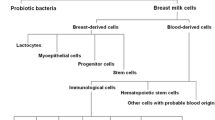
There is a diverse population of stem cells in human breast milk that can be employed for therapeutic purposes as a reservoir of cells. The current study mainly aimed to determine the nature markers expressing on stem cells. For this aim, the expression of embryonic stem cell markers, as well as the expression of endothelial, mesenchymal, neural, and hematopoietic markers were evaluated by the flow cytometry analysis in fresh colostrum, breast milk, and cultured colostrum samples. The results showed that the embryonic (OCT4, SOX2, HLA-DR), hematopoietic (CD33, CD45, CD117), neural (CD133, Nestin), and mesenchymal (CD44, SCA1) stem cell markers present in colostrum had higher expression in comparison with their counterpart markers in fresh breast milk. The expression markers of stem cells in colostrum following a 2-week culture period were significantly increased compared with their counterpart markers in colostrum before the culture process. In the culture of breastmilk, cells were not observed adherent cells and colonies. Our findings form flow cytometry and cell culture suggest that the lactation stage could be one of the factors influencing the stem cell population and, consequently, the cultivation of breastmilk cells. The present study indicates that colostrum is a tremendous source of stem cells that could be applied in cell-based research.






Similar content being viewed by others
References
Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30(9):681–7.
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–18.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Wang JM, Gu Y, Pan CJ, Yin LR. Isolation, culture and identification of human adipose-derived stem cells. Exp Ther Med. 2017;13(3):1039–43.
Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287(2):289–300.
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell–enriched human hair follicle bulge cells. J Clin Investig. 2006;116(1):249–60.
Yang C-C, Shih Y-H, Ko M-H, Hsu S-Y, Cheng H, Fu Y-S. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336.
Hartmann PE. The lactating breast: an overview from down under. Breastfeed Med. 2007;2(1):3–9.
Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat. 2013;26(1):29–48.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70.
Böcker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Investig. 2002;82(6):737.
DeOme K, Faulkin L, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19(5):515.
Gudjonsson T, Villadsen R, Nielsen HL, Rønnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16(6):693–706.
Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007;329(1):129–36.
Patki S, Kadam S, Chandra V, Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell. 2010;23(2):35–40.
Fan Y, Chong YS, Choolani MA, Cregan MD, Chan JK. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS One. 2010;5(12):e14421.
Indumathi S, Dhanasekaran M, Rajkumar J, Sudarsanam D. Exploring the stem cell and non-stem cell constituents of human breast milk. Cytotechnology. 2013;65(3):385–93.
Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30(10):2164–74.
Hosseini SM, Talaei-Khozani T, Sani M, Owrangi B. Differentiation of human breast-milk stem cells to neural stem cells and neurons. Neurol Res Int. 2014. https://doi.org/10.1155/2014/807896.
Sani M, Hosseini SM, Salmannejad M, Aleahmad F, Ebrahimi S, Jahanshahi S, et al. Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol Int. 2015;39(5):611–8.
Hassiotou F, Geddes DT, Hartmann PE. Cells in human milk: state of the science. J Hum Lact. 2013;29(2):171–82.
Ninkina N, Kukharsky MS, Hewitt MV, Lysikova EA, Skuratovska LN, Deykin AV, et al. Stem cells in human breast milk. Hum Cell. 2019;32:223–30.
Hassiotou F, Hepworth AR, Metzger P, Tat Lai C, Trengove N, Hartmann PE, et al. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin Transl Immunol. 2013;2(4):e3.
Twigger A-J, Hepworth AR, Lai CT, Chetwynd E, Stuebe AM, Blancafort P, et al. Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci Rep. 2015;5:12933.
Keller T, Wengenroth L, Smorra D, Probst K, Kurian L, Kribs A, et al. Novel DRAQ5™/SYTOX® blue based flow cytometric strategy to identify and characterize stem cells in human breast milk. Cytometry B Clin Cytom. 2019;96:480–9.
Abd Allah SH, Shalaby SM, El-Shal AS, El Nabtety SM, Khamis T, Abd El Rahman SA, et al. Breast milk MSCs: an explanation of tissue growth and maturation of offspring. IUBMB Life. 2016;68(12):935–42.
Hatori M, Shimozawa N, Yasmin L, Suemori H, Nakatsuji N, Ogura A, et al. Role of retinoic acid and fibroblast growth factor 2 in neural differentiation from cynomolgus monkey (Macaca fascicularis) embryonic stem cells. Comp Med. 2014;64(2):140–7.
Mong J, Panman L, Alekseenko Z, Kee N, Stanton LW, Ericson J, et al. Transcription factor-induced lineage programming of noradrenaline and motor neurons from embryonic stem cells. Stem Cells. 2014;32(3):609–22.
Nistor G, Siegenthaler MM, Poirier SN, Rossi S, Poole AJ, Charlton ME, et al. Derivation of high purity neuronal progenitors from human embryonic stem cells. PLoS One. 2011;6(6):e20692.
Vazey EM, Dottori M, Jamshidi P, Tomas D, Pera MF, Horne M, et al. Comparison of transplant efficiency between spontaneously derived and noggin-primed human embryonic stem cell neural precursors in the quinolinic acid rat model of Huntington’s disease. Cell Transpl. 2010;19(8):1055–62. https://doi.org/10.3727/096368910x494632.
Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9(1):36.
Lee MW, Park YJ, Kim DS, Park HJ, Jung HL, Lee JW, et al. Human adipose tissue stem cells promote the growth of acute lymphoblastic leukemia cells in NOD/SCID mice. Stem Cell Rev Rep. 2018;14(3):451–60.
Mohyeddin Bonab M, Ali Sahraian M, Aghsaie A, Ahmadi Karvigh S, Massoud Hosseinian S, Nikbin B, et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. 2012;7(6):407–14.
Acknowledgements
This research was granted by the Iran University of Medical Sciences (with the grant number IR.IUMS.REC; 1395/9221313202) and Hormozgan University of Medical Sciences (with the grant number 94007).
Author information
Authors and Affiliations
Contributions
MF and KM participated in the conception and design of the study. GN, ME, AB, ED, and GV isolated and prepared and performed flow cytometry analysis. GN, SR, MF, and SM wrote the first draft. All authors read and revised the manuscript. Additionally, all authors confirmed the final version of the manuscript to be submitted.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript. The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the current study was approved by the Ethics Committee of the Iran University of Medical Sciences.
Informed consent
The project aims and procedures were explained to volunteers, and they were asked to sign the written consent forms before participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goudarzi, N., Shabani, R., Ebrahimi, M. et al. Comparative phenotypic characterization of human colostrum and breast milk-derived stem cells. Human Cell 33, 308–317 (2020). https://doi.org/10.1007/s13577-019-00320-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00320-x