Abstract
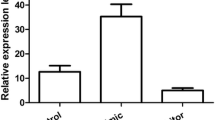
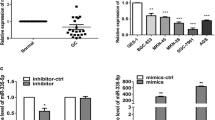
Gastric cancer is one of the most common aggressive malignancies with high incidence and mortality. Increasing evidence has suggested that microRNAs (miRNAs) are involved in the initiation and development of gastric cancer. Here, we found that miR-1179 was significantly down-regulated in both gastric cancer tissues and cell lines. Decreased expression of miR-1179 was remarkably correlated with the increased tumor size, higher tumor stage and lymph node metastasis of gastric cancer patients. Overexpression of miR-1179 significantly inhibited the proliferation and invasion of gastric cancer cells. Further molecular experiments showed that miR-1179 bound the 3′-untranslated region of the high mobility group box 1 (HMGB1) and decreased the expression of HMGB1 in gastric cancer cells. The level of HMGB1 was negatively correlated with the expression of miR-1179 in gastric cancer tissues. Rescue experiment demonstrated that restore the expression of HMGB1 significantly inversed the inhibitory effect of miR-1179 on the proliferation of gastric cancer cells. Our results uncovered the novel function of miR-1179/HMGB1 axis in the progression of gastric cancer.




Similar content being viewed by others
References
Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(24):e6884. https://doi.org/10.1097/MD.0000000000006884.
Coburn N, Cosby R, Klein L, Knight G, Malthaner R, Mamazza J, et al. Staging and surgical approaches in gastric cancer: a systematic review. Cancer Treat Rev. 2018;63:104–15. https://doi.org/10.1016/j.ctrv.2017.12.006.
Shimizu D, Kanda M, Kodera Y. Review of recent molecular landscape knowledge of gastric cancer. Histol Histopathol. 2018;33(1):11–26. https://doi.org/10.14670/HH-11-898.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–49. https://doi.org/10.3748/wjg.v20.i7.1635.
Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. https://doi.org/10.1177/1010428317714626.
Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. https://doi.org/10.1055/s-0034-1397344.
Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. https://doi.org/10.1146/annurev-biochem-060308-103103.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5. https://doi.org/10.1038/nature02871.
Xie M, Ma L, Xu T, Pan Y, Wang Q, Wei Y, et al. Potential regulatory roles of microRNAs and long noncoding RNAs in anticancer therapies. Mol Ther Nucleic Acids. 2018;13:233–43. https://doi.org/10.1016/j.omtn.2018.08.019.
Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101(11):2309–15. https://doi.org/10.1111/j.1349-7006.2010.01683.x.
Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–15. https://doi.org/10.1002/path.2806.
Qu H, Xu W, Huang Y, Yang S. Circulating miRNAs: promising biomarkers of human cancer. Asian Pac J Cancer Prev APJCP. 2011;12(5):1117–25.
Gentilin E, Degli Uberti E, Zatelli MC. Strategies to use microRNAs as therapeutic targets. Best Pract Res Clin Endocrinol Metab. 2016;30(5):629–39. https://doi.org/10.1016/j.beem.2016.10.002.
Yu H, Zhang J, Wen Q, Dai Y, Zhang W, Li F, et al. MicroRNA-6852 suppresses cell proliferation and invasion via targeting forkhead box J1 in gastric cancer. Exp Ther Med. 2018;16(4):3249–55. https://doi.org/10.3892/etm.2018.6569.
Xu J, Wang F, Wang X, He Z, Zhu X. miRNA-543 promotes cell migration and invasion by targeting SPOP in gastric cancer. Onco Targets Ther. 2018;11:5075–82. https://doi.org/10.2147/OTT.S161316.
Park SK, Park YS, Ahn JY, Do EJ, Kim D, Kim JE, et al. MiR 21-5p as a predictor of recurrence in young gastric cancer patients. J Gastroenterol Hepatol. 2016;31(8):1429–35. https://doi.org/10.1111/jgh.13300.
Kao HW, Pan CY, Lai CH, Wu CW, Fang WL, Huang KH, et al. Urine miR-21-5p as a potential non-invasive biomarker for gastric cancer. Oncotarget. 2017;8(34):56389–97. https://doi.org/10.18632/oncotarget.16916.
Li Q, Li B, Li Q, Wei S, He Z, Huang X, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9(9):854. https://doi.org/10.1038/s41419-018-0928-8.
Song L, Dai Z, Zhang S, Zhang H, Liu C, Ma X, et al. MicroRNA-1179 suppresses cell growth and invasion by targeting sperm-associated antigen 5-mediated Akt signaling in human non-small cell lung cancer. Biochem Biophys Res Commun. 2018;504(1):164–70. https://doi.org/10.1016/j.bbrc.2018.08.149.
Lin C, Hu Z, Yuan G, Su H, Zeng Y, Guo Z, et al. MicroRNA-1179 inhibits the proliferation, migration and invasion of human pancreatic cancer cells by targeting E2F5. Chemico-Biol Interact. 2018;291:65–71. https://doi.org/10.1016/j.cbi.2018.05.017.
Xu X, Cai N, Zhi T, Bao Z, Wang D, Liu Y, et al. MicroRNA-1179 inhibits glioblastoma cell proliferation and cell cycle progression via directly targeting E2F transcription factor 5. Am J Cancer Res. 2017;7(8):1680–92.
Kumari T, Kumar B. High-mobility group box 1 protein (HMGB1) gene polymorphisms and cancer susceptibility: a comprehensive meta-analysis. Clin Chim Acta. 2018;483:170–82. https://doi.org/10.1016/j.cca.2018.04.042.
Huang CY, Chiang SF, Ke TW, Chen TW, Lan YC, You YS, et al. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1 + TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother. 2018;67(4):551–62. https://doi.org/10.1007/s00262-017-2109-5.
Xu Y, Chen Z, Zhang G, Xi Y, Sun R, Chai F, et al. HMGB1 overexpression correlates with poor prognosis in early-stage squamous cervical cancer. Tumour Biol. 2015;36(11):9039–47. https://doi.org/10.1007/s13277-015-3624-7.
Wu T, Zhang W, Yang G, Li H, Chen Q, Song R, et al. HMGB1 overexpression as a prognostic factor for survival in cancer: a meta-analysis and systematic review. Oncotarget. 2016;7(31):50417–27. https://doi.org/10.18632/oncotarget.10413.
Chen J, Li G. MiR-1284 enhances sensitivity of cervical cancer cells to cisplatin via downregulating HMGB1. Biomed Pharmacother. 2018;107:997–1003. https://doi.org/10.1016/j.biopha.2018.08.059.
Lu L, Zhang D, Xu Y, Bai G, Lv Y, Liang J. miR-505 enhances doxorubicin-induced cytotoxicity in hepatocellular carcinoma through repressing the Akt pathway by directly targeting HMGB1. Biomed Pharmacother. 2018;104:613–21. https://doi.org/10.1016/j.biopha.2018.05.087.
Tian L, Wang ZY, Hao J, Zhang XY. miR-505 acts as a tumor suppressor in gastric cancer progression through targeting HMGB1. J Cell Biochem. 2018. https://doi.org/10.1002/jcb.28082.
Wu D, Liu J, Chen J, He H, Ma H, Lv X. MiR-449a suppresses tumor growth, migration and invasion in non-small cell lung cancer by targeting HMGB1-mediated NF-kappaB signaling way. Oncol Res. 2018. https://doi.org/10.3727/096504018X15213089759999.
Feng XJ, Liu SX, Wu C, Kang PP, Liu QJ, Hao J, et al. The PTEN/PI3K/Akt signaling pathway mediates HMGB1-induced cell proliferation by regulating the NF-kappaB/cyclin D1 pathway in mouse mesangial cells. Am J Physiol Cell Physiol. 2014;306(12):C1119-28. https://doi.org/10.1152/ajpcell.00385.2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13577_2019_244_MOESM1_ESM.tif
Supplementary material 1 Supplementary Figure 1 Overexpression of miR-1179 decreased the expression of HMGB1 and inhibited the growth of MGC-803 and MKN-45 cells. a, b Overexpression of miR-1179 suppressed the proliferation of both MGC-803 and MKN-45 cells. c, d Transfection of miR-1179 inhibited the mRNA and protein expression of HMGB1 in MGC-803 and MKN-45 cells (TIF 1563 KB)
Rights and permissions
About this article
Cite this article
Li, Y., Qin, C. MiR-1179 inhibits the proliferation of gastric cancer cells by targeting HMGB1. Human Cell 32, 352–359 (2019). https://doi.org/10.1007/s13577-019-00244-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00244-6