Abstract
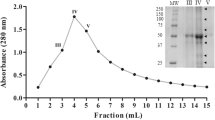
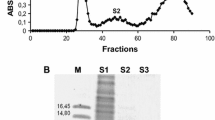
Seed proteins recovered after heating a seed extract from Opuntia joconostle Weber (xoconostle, an acid cactus pear) were screened for different biochemical activities, detecting only trypsin-like inhibitory activity. Two trypsin-like inhibitor forms from seeds were separated by RP-HPLC and partially sequenced and characterized as an enriched mixture. They were evaluated for inhibition on several serine proteinases, but only trypsin-like inhibition was detected by the inhibitor extract. The two isolated forms, OjTI 1 and OjTI 2 showed low molecular weights of 4.26 and 4.17 kDa as determined by mass spectrometry. An enriched inhibitory fraction showed a high thermal stability by retaining the activity after heating the sample for 1 h at 90 °C, as well as after heating for at 120 °C under 1 kg/cm2 for 15 min at different pH values. Partial sequence of the two forms was determined by mass spectrometry indicating that they were similar and after alignments analysis they showed the highest similarity with the trypsin inhibitor from O. streptacantha and to a lesser extent to other trypsin inhibitors of the MEROPS database families. The inhibitory spectrum was evaluated against several digestive enzymes from pests and beneficial insects from several taxonomic orders.


Similar content being viewed by others
Abbreviations
- PIs:
-
Proteinase inhibitors
- OjTIs:
-
Opuntia joconostle trypsin inhibitors
References
Abdeen A, Virgos A, Olivella E, Villanueva J, Aviles X, Gabarra R, Prat S (2005) Multiple insect resistance in transgenic tomato plants over-expressing two families of plant proteinase inhibitors. Plant Mol Biol 57(2):189–202
Azzouz H, Campan E, Cherqui A, Saguez J, Couty A, Jouanin L, Kaiser L, Giordanengo P (2005) Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis(Hymenoptera, Aphelinidae). J Insect Physiol 51(8):941–951
Conners R, Konarev AV, Forsyth J, Lovegrove A, Marsh J, Joseph-Horne T, Shewry P, Brady RL (2007) An unusual helix-turn-helix protease inhibitory motif in a novel trypsin inhibitor from seeds of veronica (Veronica hederifolia L.). J Biol Chem 282(38):27760–27768
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95(2):271–278
Hou W, Lin YH (2002) Sweet potato (Ipomoea batatas (L.) Lam) trypsin inhibitors, the major root storage proteins, inhibit one endogenous serine protease activity. Plant Sci 163:733–739
Hou W, Han CH, Chen HJ, Wen CL, Lin YH (2005) Storage proteins of two cultivars of sweet potato (Ipomoea batatas L.) and their protease hydrolysates exhibited antioxidant activity in vitro. Plant Sci 168:449–456
Huang YM, Xiao BL, Xiong LZ (2007) Characterization of a stress responsive proteinase inhibitor gene with positive effect in improving drought resistance in rice. Planta 226:73–85
Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahn KS, Park Y (2009) Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci 10:2960–2872
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Konarev AV, Tomooka N, Vaughan DA (2002) Proteinase inhibitor polymorphism in the genus Vigna subgenus Ceratotropis and its biosystematic implications. Euphytica 123:165–177
Konarev A, Griffin J, Konechnaya GY, Shewry P (2004) The distribution of serine proteinase inhibitors in seeds of Asteridae. Phytochemistry 65:3003–3020
Lawrence PK, Koundal KR (2002) Plant protease inhibitors in control of phytophagous insects. Electron J Biotechn 5:1–17
Maity J, Patra B (2003) Isolation and characterization of trypsin inhibitor from the water fern, Azolla pinnata. R Br J Food Biochem 27(3):281–294
Major IT, Constabel CP (2008) Functional analysis of the kunitz trypsin inhibitor family in poplar reveals biochemical diversity and multiplicity in defense against herbivores. Plant Physiol 146:888–903
Mello MO, Tanaka AS, Silva-Filho MC (2003) Molecular evolution of Bowman-Birk type proteinase inhibitors in flowering plants. Mol Phylogenet Evol 27(1):103–112
Oliva MV, Sampaio MU (2009) Action of plant proteinase inhibitors on enzymes of physiopathological importance. An Acad Bras Cienc 81(3):615–621
Qi RF, Song ZW, Chi CW (2005) Structural features and molecular evolution of Bowman-Birk protease inhibitors and their potential application. Acta Bioch Bioph Sin 37:283–292
Rawlings ND, Tolle DP, Barrett AJ (2004) Evolutionary families of peptidase inhibitors. Biochem J 378:705–716
Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ (2008) MEROPS: the peptidase database. Nucl Acid Res 36(database issue):320–325
Schägger H, Von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 66:368–379
Torres-Castillo JA, Mondragón-Jacobo C, Blanco-Labra A (2009) Characterization of a highly stable trypsin-like ´proteinase inhibitor from the seeds of Opuntia streptacantha (O. streptacantha Lemaire). Phytochemistry 70(11–12):1374–1381
Valueva TA, Mosolov VV (2004) Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochem Mosc 69:1305–1309
Yeh K, Lin M, Tuan S, Chen Y, Lin C, Kao S (1996) Sweet potato (Ipomoea batatas) trypsin inhibitors expressed in transgenic tobacco plants confer resistance against Spodoptera litura. Plant Cell Rep 16(10):696–699
Zavala JA, Patankar AG, Gase K, Hui D, Baldwin IT (2004) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134:1181–1190
Acknowledgment
We would like to thank PROMEP for economical support to the project PROMEP/103.5/10/7303 for the scholarship for Aguirrezabala-Cámpano María Teresa and financial support of this research. Also we want to thank to the PAICTY-UANL 2010 budget for financial support of project 47. Also, we want to thank Yolanda Rodríguez Aza and Ma. Cristina Elizarraráz Anaya for their invaluable help and technical support during the purification of the inhibitor through HPLC. Finally, we thank to Biotechnology Laboratory from the Faculty of Agronomy from UANL for the facilities provided to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguirrezabala-Cámpano, M.T., Torres-Acosta, R.I., Blanco-Labra, A. et al. Trypsin inhibitors in xoconostle seeds (Opuntia joconostle Weber.). J. Plant Biochem. Biotechnol. 22, 261–268 (2013). https://doi.org/10.1007/s13562-012-0152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-012-0152-z