Abstract
Introduction
At first interim analysis of KEYNOTE-629, health-related quality of life (HRQoL) with pembrolizumab was stable or improved over 48 weeks in recurrent or metastatic (R/M) cutaneous squamous cell carcinoma (cSCC). HRQoL results from the second interim analysis in R/M or locally advanced (LA) cSCC are presented.
Methods
Patients received pembrolizumab 200 mg every 3 weeks for ≤ 2 years. Change in EORTC Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and EQ-5D-5L scores were exploratory end points. Primary analysis was performed at week 12 to ensure adequate completion/compliance. Descriptive analyses were also conducted through weeks 48 and 75 for the LA and R/M cohorts, respectively.
Results
At data cutoff (29 July 2020), mean scores in the LA cohort (n = 47) were stable from baseline to week 12 for EORTC QLQ-C30 global health status (GHS)/quality of life (QoL) (−0.27 points [95% confidence interval (CI) −10.93 to 10.39]), physical functioning (−1.29 points [95% CI −8.77 to 6.19]), and EQ-5D-5L visual analog scale (2.06 [95% CI −7.70 to 11.82]). HRQoL remained stable through week 48 in the LA cohort; 76.6% and 74.5% of patients had improved or stable GHS/QoL and physical functioning scores, respectively. HRQoL continued to show stability or improvement through week 75 in the R/M cohort (n = 99); 71.7% and 64.6% of patients had improved or stable GHS/QoL and physical functioning scores, respectively.
Conclusions
Pembrolizumab has demonstrated antitumor activity and manageable safety. The current analysis shows pembrolizumab treatment preserved HRQoL. Collectively, these results support pembrolizumab as standard of care for LA or R/M cSCC.
Trial Registration
ClinicalTrials.gov, NCT03284424—September 15, 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The symptoms and treatment outcomes of patients with advanced cutaneous squamous cell carcinoma (cSCC) can have a marked effect on their health-related quality of life (HRQoL). |
Assessment of HRQoL is an important part of assessing new therapies. |
Prespecified analysis of HRQoL in patients with recurrent/metastatic (R/M) cSCC at the first interim analysis of KEYNOTE-629 showed that HRQoL was stable with pembrolizumab and that improvement in HRQoL was positively correlated with treatment response; HRQoL results from the second interim analysis in R/M or locally advanced (LA) cSCC are presented. |
Results show that pembrolizumab treatment preserved HRQoL. |
Together with data showing pembrolizumab has antitumor activity and manageable safety in patients with LA or R/M cSCC, the HRQoL results support pembrolizumab as a standard-of-care treatment option for patients with LA or R/M cSCC not curable by surgery or radiotherapy. |
Introduction
Cutaneous squamous cell carcinoma (cSCC) is a common type of nonmelanoma skin cancer (NMSC) resulting from uncontrolled proliferation of epidermal keratinocytes [1, 2]. While traditionally considered to account for 20% of cutaneous malignancies, the true incidence of cSCC is largely unknown because it is often excluded from cancer registries or the incidence is reported in combination with basal cell carcinoma as NMSC [1,2,3]. The etiology of cSCC is multifactorial, with the main risk factors including cumulative exposure to ultraviolet (UV) radiation, fair phototype, and age [1, 2, 4]. The incidence of cSCC is therefore higher in countries with ozone depletion, sun-seeking behavior, and predominantly White (per AMA) populations [4].
In most cases, surgical resection of cSCC is curative, but a small proportion of patients develop recurrent/metastatic (R/M) or locally advanced (LA) cSCC, which generally requires systemic treatment and has a poor prognosis [5, 6]. Because of the high tumor mutational burden associated with UV-mediated carcinogenesis, cSCC is an immunogenic cancer that is amenable to immunotherapy [7, 8]. Consequently, the current standard of care for patients with R/M or LA cSCC not curable by surgery and radiotherapy is treatment with the anti-programmed death 1 antibodies cemiplimab or pembrolizumab [9,10,11,12].
The symptoms and treatment outcomes of patients with advanced cSCC can have a marked effect on their health-related quality of life (HRQoL) [6]. Patients may experience scarring and disfigurement and impairment in functions such as speech and swallowing [6, 13]. Pain is also a significant and common feature of cSCC [14]. Assessment of HRQoL is therefore an important part of assessing new therapies.
In the phase 2 KEYNOTE-629 study, pembrolizumab demonstrated robust antitumor activity and a favorable safety profile in a relatively fragile study population composed primarily of elderly and heavily pretreated patients with LA or R/M cSCC [15, 16]. Prespecified analysis of HRQoL in patients with R/M cSCC at the first interim analysis (data cutoff, 8 April 2019) showed that HRQoL was stable with pembrolizumab and that improvement in HRQoL was positively correlated with treatment response [17]. We present HRQoL results from the second interim analysis of KEYNOTE-629 (data cutoff, 29 July 2020), which represents updated results for patients with R/M cSCC and the first report of HRQoL data for patients with LA cSCC.
Methods
Study Design
KEYNOTE-629 (NCT03284424) is an ongoing multisite, open-label, nonrandomized, single-arm, phase 2 study of pembrolizumab in patients with unresectable LA or R/M cSCC. Detailed methods and eligibility criteria for KEYNOTE-629 have been published previously [15, 16]. Briefly, eligible patients were ≥ 18 years old, had histologically confirmed LA or R/M cSCC, and had measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1. Patients in the R/M cohort were required to have locally recurrent disease not curable by surgery or radiation or metastatic disease. Patients in the LA cohort were required to be ineligible for surgical resection and must have undergone prior radiation therapy or be ineligible for radiotherapy. Eligible patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate organ function.
Patients received pembrolizumab 200 mg intravenously every 3 weeks until disease progression, unacceptable toxicity, or study withdrawal for a maximum of 35 cycles (approximately 2 years). Patient-reported outcomes (PROs) questionnaires were administered electronically before pembrolizumab administration at baseline, week 3, and week 6, then every 6 weeks for the remainder of the first 12 months and every 9 weeks thereafter. HRQoL was assessed using the EORTC Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and EQ-5D-5L instruments, both of which have been extensively validated and widely used in cancer studies. The EORTC QLQ-C30 instrument includes a global health status (GHS)/quality of life (QoL) scale, five functional subscales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, pain, and nausea), and six single-item scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) [18]. The EQ-5D-5L instrument includes a descriptive system comprising five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) scored using five response levels and a visual analog scale (VAS) [19]. A detailed description of these instruments and their utility in the cSCC setting has been published previously [17].
HRQoL End Points
The prespecified exploratory HRQoL end point was change from baseline in EORTC QLQ-C30 GHS/QoL, functioning, and symptom scores and EQ-5D-5L scores. The primary analysis was conducted at week 12 or at the latest time point at which completion rate was approximately 60% or more and compliance rate was approximately 80% or more. Mean change from baseline in EORTC QLQ-C30 GHS/QoL and physical functioning scores were also summarized in patients who were on study and able to complete the questionnaire through week 48 for the LA cohort and week 75 for the R/M cohort.
Responses for each of the EORTC QLQ-C30 scales (GHS/QoL, functioning subscales, symptom subscale, and single-item scores) were calculated by averaging items within scales and linearly transforming the scores so that they ranged from 0 to 100. Clinically meaningful differences in EORTC QLQ-C30 scales were defined as a change of ≥ 10 points from baseline [20]. Overall improvement was defined as a ≥ 10-point increase in score from baseline at any time during the trial, with confirmation at the next visit. For patients who did not achieve improved HRQoL scores, stable scores were defined as any of the following: improvement (a ≥ 10-point increase in score) confirmed by a < 10-point change in score at the next visit, < 10-point change in score confirmed by a < 10-point change at the next visit, or a < 10-point change in score confirmed by an improvement at the next visit. Deterioration was defined as a ≥ 10-point decrease in score from baseline at any time during the trial for patients without improved or stable scores. Data are presented as the proportion of patients who meet these definitions through week 48 for the LA cohort and week 75 for the R/M cohort.
For the EQ-5D-5L VAS, responses were scored from 0 (worst health imaginable) to 100 (best health imaginable), and clinically meaningful differences relative to baseline were defined as a change of ≥ 7 points [19, 21].
The PRO compliance rate was defined as the proportion of patients who completed ≥ 1 PRO assessment among those who were expected to complete HRQoL assessments at a given time point, excluding patients missing by design (e.g., those who had died or discontinued the study). The PRO completion rate was defined as the proportion of patients who completed ≥ 1 PRO assessment among all patients in the HRQoL analysis population.
Statistical Analysis
PRO analyses included all patients who had both baseline and ≥ 1 postbaseline PRO assessment available and had received ≥ 1 dose of study treatment. All HRQoL analyses were descriptive. The data cutoff was 29 July 2020 (interim analysis 2).
Ethics Approval and Consent to Participate
The study protocol and amendments were approved by the appropriate institutional review boards and ethics review committees at each institution (Supplementary Material Table 1). The study was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the Declaration of Helsinki. All participants provided written informed consent.
Results
Patient Disposition, Baseline Characteristics, and Follow-Up
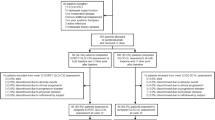
A total of 159 patients were allocated to receive study treatment across 48 study sites in ten countries; 54 patients had LA cSCC and 105 patients had R/M cSCC. The HRQoL population included 47 patients from the LA cohort for both the EORTC QLQ-C30 and EQ-5D-5L instruments, 99 patients from the R/M cohort for the EORTC QLQ-C30 instrument, and 100 patients from the R/M cohort for the EQ-5D-5L instrument. Disposition for the LA HRQoL population is shown in Fig. 1. Disposition for the R/M HRQoL population has been published previously [17]. The only change in the disposition of patients with R/M cSCC at the second interim analysis was that one patient who was classified as being excluded from the week 12 EORTC QLQ-C30 assessment because of discontinuing due to clinical progression (defined as worsening of clinical status with or without radiographic progression of disease) at first interim analysis was subsequently classified as discontinuing due to progressive disease (defined as radiographically diagnosed disease progression per Response Evaluation Criteria in Solid Tumors, version 1.1). The median age of patients in the LA HRQoL population was 75.0 years, and 76.6% (36/47) of patients were ≥ 65 years (Supplementary Material Table 2). Baseline characteristics for patients in the R/M HRQoL population have been published previously [17]. The median time from first pembrolizumab dose to data cutoff was 15.3 months (range 10.1–19.4 months) for the LA cohort and 27.2 months (range 24.6–32.0 months) for the R/M cohort.
Patient disposition in the HRQoL populations for the LA cSCC cohort. aReasons for ineligibility included not meeting inclusion criteria about the following: having metastatic and/or unresectable cSCC not curable by surgery or radiation (n = 8); ineligible for surgical resection (n = 4); measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (n = 4); adequate organ function (n = 4); prior systemic therapy for curative intent (n = 2); adequate tissue sample (n = 2); provide informed consent (n = 2); aged at least 18 years (n = 1); cSCC as the primary site of malignancy (n = 1); previous radiotherapy or was ineligible for radiotherapy (n = 1); have metastatic disease, defined as disseminated disease distant to the initial/primary site of diagnosis, and/or have locally recurrent disease that had been previously treated (with either surgery or radiotherapy) that was not curable by either surgery or radiotherapy (n = 1); Eastern Cooperative Oncology Group performance status was 0 or 1 (n = 1); adequate contraception (n = 1); and/or meeting exclusion criteria about the following: have history of or current evidence of a condition that may have confounded results (n = 3), have other histologic type of skin cancer other than invasive squamous cell carcinoma (n = 2), has immunodeficiency or had received immunosuppressive therapy within 7 days of first dose of study drug (n = 3), has received prior systemic anticancer therapy within 4 weeks before allocation (n = 1), and additional malignancy (n = 3), active infection requiring systemic therapy (n = 1). Patients may have been excluded for more than one reason. One patient did not meet inclusion criteria for the R/M cohort at interim analysis 1 but was enrolled in the LA cohort. bClinical progression was defined as worsening of clinical status with or without radiographic progression of disease. cSCC cutaneous squamous cell carcinoma, LA locally advanced, R/M recurrent/metastatic, EORTC QLQ-C30 EORTC Quality of Life Questionnaire Core 3, HRQoL health-related quality of life
HRQoL Assessment Compliance and Completion
In the LA HRQoL population, compliance rates were 75.6% (31/41) for EORTC QLQ-C30 and 78.0% (32/41) for EQ-5D-5L at week 12 (Table 1). At week 48, they had increased to 85.0% (17/20) for both EORTC QLQ-C30 and EQ-5D-5L as the population of patients expected to complete the assessments decreased because of treatment discontinuation (primarily due to disease progression, adverse events, or death). In the R/M HRQoL population, compliance rates were 81.2% (69/85) for EORTC QLQ-C30 and 82.4% (70/85) for EQ-5D-5L at week 12 [17], 86.0% (37/43) and 88.4% (38/43) at week 48, and 78.4% (29/37) and 81.1% (30/37) at week 75 (Table 1).
For the LA HRQoL population, completion rates were 66.0% (31/47) for EORTC QLQ-C30 and 68.1% (32/47) for EQ-5D-5L at week 12 and 36.2% (17/47) for both instruments at week 48 (Table 1). For the R/M HRQoL population, completion rates were 69.7% (69/99) for EORTC QLQ-C30 and 70.0% (70/100) for EQ-5D-5L at week 12 [17], 37.4% (37/99) and 38.0% (38/100) at week 48, and 29.3% (29/99) and 30.0% (30/100) at week 75 (Table 1).
Mean Change from Baseline in EORTC QLQ-C30 Scores
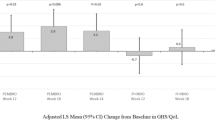
Over the first 12 weeks of follow-up, patients in the LA HRQoL population exhibited stable EORTC QLQ-C30 scores (Table 2). At week 12, the mean change from baseline was −0.27 points (95% CI −10.93 to 10.39) in EORTC QLQ-C30 GHS/QoL score and −1.29 points (95% CI −8.77 to 6.19) in EORTC QLQ-C30 physical functioning score. The EORTC QLQ-C30 GHS/QoL and physical functioning scores remained stable through week 48 (Fig. 2A). Patients in the LA HRQoL population also generally exhibited stable scores for the other functioning and symptom subscales of the EORTC QLQ-C30 (Fig. 3). A clinically meaningful improvement in the pain symptom score was observed at week 12 (mean change from baseline, −11.83 points [95% CI −0.21 to −23.45]) (Fig. 3).
Mean change from baseline for EORTC QLQ-C30 GHS/QoL and physical functioning scoresa in A the LA cSCC cohort and B the R/M cSCC cohort. aFor EORTC QLQ-C30 GHS/QoL and all functional scales, a higher score denotes better HRQoL or function. CI confidence interval, cSCC cutaneous squamous cell carcinoma, EORTC QLQ-C30 EORTC Quality of Life Questionnaire Core 30, GHS/QoL global health status/quality of life, HRQoL health-related quality of life, LA locally advanced, R/M recurrent/metastatic
Mean change from baseline to week 12 for patients with nonmissing assessment in the LA cSCC cohort at both baseline and week 12 for EORTC QLQ-C30 A GHS/QoL and functioning scalesa and B symptom scales.b aFor EORTC QLQ-C30 GHS/QoL and all functional scales, a higher score denotes better HRQoL or function. bFor EORTC QLQ-C30 symptoms scales, a higher score denotes worse symptoms. CI confidence interval, cSCC cutaneous squamous cell carcinoma, EORTC QLQ-C30 EORTC Quality of Life Questionnaire Core 30, GHS/QoL global health status/quality of life, HRQoL health-related quality of life, LA locally advanced, R/M recurrent/metastatic, HRQoL health-related quality of life
The mean change in EORTC QLQ-C30 scores from baseline to weeks 12 and 48 for the R/M HRQoL population was reported previously [17]. In the current analysis, EORTC QLQ-C30 GHS/QoL and EORTC QLQ-C30 physical functioning scores remained stable through week 75 (Fig. 2B).
Overall Improvement, Stability, and Deterioration Rate in EORTC QLQ-C30 GHS/QoL and Physical Functioning Scores over Time
Most patients in the LA HRQoL population experienced stable or improved EORTC QLQ-C30 GHS/QoL and physical functioning scores relative to baseline during follow-up (Table 3). The proportion of patients in the LA population with improved EORTC QLQ-C30 GHS/QoL scores after baseline was 42.6% (95% CI 28.3–57.8%), and 34.0% (95% CI 20.9–49.3%) exhibited stable scores (Table 3). The proportion of patients with improved EORTC QLQ-C30 physical functioning scores compared with baseline scores was 14.9% (95% CI 6.2–28.3%) and the proportion with stable scores was 59.6% (95% CI 44.3–73.6%) (Table 3).
The effect of treatment on EORTC QLQ-C30 GHS/QoL and physical functioning scores for patients in the R/M HRQoL population through week 48 has been reported previously [17]. The updated results through week 75 are presented in Table 3. The proportion of patients in the R/M population with improved GHS/QoL scores compared with baseline scores was 29.3% (95% CI 20.6–39.3%) and the proportion with stable scores was 42.4% (95% CI 32.5–52.8%) (Table 3). The proportion of patients with improved physical functioning scores compared with baseline scores was 18.2% (95% CI 11.1–27.2%), and 46.5% (95% CI 36.4–56.8%) exhibited stable scores (Table 3).
In the total HRQoL population including both LA and R/M cSCC cohorts, most patients experienced improved or stable EORTC QLQ-C30 GHS/QoL and physical functioning scores relative to baseline (Supplementary Material Table 3). The proportion of patients in the total population with improved EORTC QLQ-C30 GHS/QoL scores compared with baseline scores was 33.6% (95% CI 26.0–41.8%), and 39.7% (95% CI 31.7–48.1%) exhibited stable scores. The proportion of patients with improved EORTC QLQ-C30 physical functioning scores compared with baseline scores was 17.1% (95% CI 11.4–24.2%) and the proportion with stable scores was 50.7% (95% CI 42.3–59.0%).
In the total HRQoL population including both LA and R/M cSCC cohorts, a greater proportion of responders to pembrolizumab treatment (patients with complete response or partial response) experienced improved or stable EORTC QLQ-C30 GHS/QoL and EORTC QLQ-C30 physical functioning scores relative to baseline compared with nonresponders (stable disease or progressive disease). The proportion of patients in the total population with improved or stable EORTC QLQ-C30 GHS/QoL scores compared with baseline scores was 89.1% (95% CI 78.8–95.5%) in responders and 59.5% (95% CI 48.3–70.1%) in nonresponders. The proportion of patients in the total population with improved or stable EORTC QLQ-C30 physical functioning scores compared with baseline scores was 84.4% (95% CI 73.1–92.2%) in responders and 53.6% (95% CI 42.4–64.5%) in nonresponders.
Mean Change from Baseline in EQ-5D-5L Scores
For patients in the LA HRQoL population, the mean change from baseline to week 12 in EQ-5D-5L VAS score was 2.06 points (95% CI −7.70 to 11.82) (Table 2). The mean change in the EQ-5D-5L VAS and utility scores from baseline to week 12 for patients in the R/M HRQoL population has been reported previously [17].
Discussion
Pembrolizumab has demonstrated effective antitumor activity and manageable safety in patients with LA and R/M cSCC [15, 16]. At the first interim analysis of KEYNOTE-629, pembrolizumab provided an objective response rate of 34.3%, and median duration of response was not reached among patients with R/M cSCC [15]. At the second interim analysis, the objective response rate was 50.0% in the LA cohort and 35.2% in the R/M cohort, and the median duration of response was not reached in either cohort [16]. Results from the first interim analysis also demonstrated that HRQoL was maintained or improved in patients with R/M cSCC [17]. Mean scores were stable from baseline to week 12 for EORTC QLQ-C30 GHS/QoL (4.95 points; 95% CI −1.00 to 10.90) and physical functioning (−3.38 points; 95% CI −8.80 to 2.04), and for EQ-5D-5L VAS (1.97 points; 95% CI −3.85 to 7.79). Postbaseline EORTC QLQ-C30 GHS/QoL and physical functioning scores were improved in 29.3% and 17.2% of patients, respectively. At the first interim analysis, a greater proportion of responders than nonresponders with R/M cSCC experienced improvements in GHS/QoL (55.6% versus 16.1%) and physical functioning (36.1% versus 7.1%) scores relative to baseline [17].
The current analysis of HRQoL in the KEYNOTE-629 study supports the previous findings in R/M cSCC and further showed that HRQoL was maintained or improved with pembrolizumab in patients with LA cSCC.
In patients with LA cSCC, pembrolizumab treatment at week 12 was associated with stable EORTC QLQ-C30 GHS/QoL and physical functioning scores and EQ-5D-5L scores. Except for a clinically meaningful improvement in pain symptom score, differences in other EORTC QLQ-C30 functioning and symptom subscales remained stable from baseline to week 12. The stability of EORTC QLQ-C30 GHS/QoL and physical functioning scores was also seen over 48 weeks; 76.6% and 74.5% of patients in the LA cohort had GHS/QoL and physical functioning scores that had not deteriorated (i.e., were improved or stable) relative to baseline, respectively. In patients with R/M cSCC, the stable EORTC QLQ-C30 GHS/QoL and physical functioning scores observed over 48 weeks in the prior analysis [17] remained stable with additional follow-up through week 75, 71.7% and 64.6% of patients in the R/M cohort had GHS/QoL and physical functioning scores that had not deteriorated (i.e., improved or stable) relative to baseline. Furthermore, descriptive analyses of the total HRQoL population (LA and R/M cohorts) showed that treatment response is positively correlated with HRQoL in that a greater proportion of responders compared with nonresponders had EORTC QLQ-C30 GHS/QoL and physical functioning scores that had not deteriorated relative to baseline.
Results from our study are comparable with the HRQoL results of a phase 2 study of cemiplimab in 193 patients with LA or R/M cSCC. The study reported an improvement in EORTC QLQ-C30 GHS/QoL from week 6, becoming clinically meaningful at week 36 [22, 23]. Improvement was seen in emotional and social functioning and nausea/vomiting, insomnia, appetite loss, constipation, and pain symptom scores. Physical, role, and cognitive functioning scores remained stable relative to baseline. In our study, although clinically meaningful improvement was observed only for the pain symptom subscale, all other functioning and symptoms subscales were stable, with no sign of clinically meaningful HRQoL deterioration. These results are particularly noteworthy given that the majority of patients with cSCC in this study were elderly and because pain is a common feature of cSCC [14, 24]. Therefore, effective treatments that maintain HRQoL without further deterioration are certainly advantageous for these patients. The primary limitation of this study is the single-arm design, which prevents comparison of HRQoL with other agents. Further, the study was open label, which may have influenced patient responses. Another limitation, which is common to studies evaluating HRQoL, was the need to conduct the primary analysis at week 12 to ensure adequate completion and compliance rates. However, the results of the longer-term follow-up showed that EORTC QLQ-C30 GHS/QoL and physical functioning scores remained stable through week 48 for patients with LA cSCC and through week 75 for patients with R/M cSCC. Despite these limitations, this analysis provides valuable information in a population who often experience significantly impacted HRQoL, in a setting in which there are limited prospective trial data available.
Conclusions
Results of this analysis showed that HRQoL is stable with pembrolizumab in patients with LA cSCC and complemented earlier findings showing that HRQoL is stable or improved in patients with R/M cSCC. Together with data showing pembrolizumab has antitumor activity and manageable safety in patients with LA or R/M cSCC, the HRQoL results support pembrolizumab as a standard-of-care treatment option for patients with LA or R/M cSCC not curable by surgery or radiotherapy.
Data Availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–47.
Fania L, Didona D, Di Pietro FR, Verkhovskaia S, Morese R, Paolino G, et al. Cutaneous squamous cell carcinoma: From pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9(2):171.
Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11(1):4337.
Stang A, Khil L, Kajüter H, Pandeya N, Schmults CD, Ruiz ES, et al. Incidence and mortality for cutaneous squamous cell carcinoma: comparison across three continents. J Eur Acad Dermatol Venereol. 2019;33(suppl 8):6–10.
Petersen ET, Ahmed SR, Chen L, Silapunt S, Migden MR. Review of systemic agents in the treatment of advanced cutaneous squamous cell carcinoma. Future Oncol. 2019;15(27):3171–84.
Starkings R, Shilling V, Jenkins V, Fallowfield L. A structured review of quality of life in advanced and high-risk cutaneous squamous cell carcinoma shows the need for more studies and better measures. Skin Health Dis. 2021;1(3): e39.
Ishitsuka Y, Hanaoka Y, Tanemura A, Fujimoto M. Cutaneous squamous cell carcinoma in the age of immunotherapy. Cancers (Basel). 2021;13(5):1148.
Boutros A, Cecchi F, Tanda ET, Croce E, Gili R, Arecco L, et al. Immunotherapy for the treatment of cutaneous squamous cell carcinoma. Front Oncol. 2021;11: 733917.
Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 2. Treatment. Eur J Cancer. 2020;128:83–102.
Keohane SG, Botting J, Budny PG, Dolan OM, Fife K, Harwood CA, et al. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma 2020. Br J Dermatol. 2021;184(3):401–14.
Villani A, Ocampo-Garza SS, Potestio L, Fabbrocini G, Ocampo-Candini J, Ocampo-Garza J, Scalvenzi M. Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma. Expert Opin Drug Saf. 2022;21(1):21–9.
Villani A, Potestio L, Fabbrocini G, Scalvenzi M. New emerging treatment options for advanced basal cell carcinoma and squamous cell carcinoma. Adv Ther. 2022;39(3):1164–78.
Wang AY, Palme CE, Wang JT, Morgan GJ, Gebski V, Gilchrist J, et al. Quality of life assessment in patients treated for metastatic cutaneous squamous cell carcinoma of the head and neck. J Laryngol Otol. 2013;127:S39–S47.
Mills KC, Kwatra SG, Feneran AN, Pearce DJ, Williford PM, D’Agostino RB, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148(12):1422–3.
Grob JJ, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II Trial (KEYNOTE-629). J Clin Oncol. 2020;38(25):2916–25.
Hughes BGM, Munoz-Couselo E, Mortier L, Bratland Å, Gutzmer R, Roshdy O, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. 2021;32(10):1276–85.
Hughes BGM, Mendoza RG, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Health-related quality of life of patients with recurrent or metastatic cutaneous squamous cell carcinoma treated with pembrolizumab in KEYNOTE-629. Dermatol Ther (Heidelb). 2021;11(5):1777–90.
Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. 2008. https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf. Accessed 16 Sept 2019.
EuroQol Research Foundation. EQ-5D-5L User Guide. Basic information on how to use the EQ-5D-5L instrument. 2019. https://euroqol.org/publications/user-guides/. Accessed 20 Feb 2020.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44.
Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
Migden MR, Rischin D, Sasane M, Mastey V, Pavlick A, Schmults CD, et al. Health-related quality of life (HRQL) in patients with advanced cutaneous squamous cell carcinoma (CSCC) treated with cemiplimab: post hoc exploratory analyses of a phase II clinical trial. J Clin Oncol. 2020;38(15_suppl):10033.
Rischin D, Khushalani NI, Schmults CD, Guminski A, Chang ALS, Lewis KD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. 2021;9(8).
Garcovich S, Colloca G, Sollena P, Andrea B, Balducci L, Cho WC, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643–61.
Acknowledgements
The authors thank the patients and their families and caregivers and all investigators and site personnel. For statistical support, the authors thank Pingye Zhang, who was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, at the time of the conduct of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Doyel Mitra, PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this research and the journal’s Rapid Service Fee was provided by Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Conception, design or planning of the study: Joy Ge, Burak Gumuscu, Ramona F. Swaby. Acquisition of the data: Åse Bratland, Eva Munoz-Couselo, Laurent Mortier, Osama Roshdy, Florent Grange, Nicolas Meyer, Salem Billan, Jean-Jacques Grob, Burak Gumuscu, Ralf Gutzmer. Analysis of the data: Åse Bratland, Rene González, Nicolas Meyer, Karthik Ramakrishnan, Joy Ge, Burak Gumuscu, Ramona F. Swaby. Interpretation of the results: Åse Bratland, Eva Munoz-Couselo, Laurent Mortier, Rene González, Jacob Schachter, Ana M. Arance, Nicolas Meyer, Salem Billan, Brett G. M. Hughes, Karthik Ramakrishnan, Joy Ge, Burak Gumuscu, Ramona F. Swaby, Ralf Gutzmer. Writing- drafting of the manuscript: Åse Bratland, Burak Gumuscu, Ramona F. Swaby. Writing- critically reviewing or revising the manuscript for important intellectual content: Åse Bratland, Eva Munoz-Couselo, Laurent Mortier, Osama Roshdy, Rene González, Jacob Schachter, Ana M. Arance, Florent Grange, Nicolas Meyer, Abhishek Jagdish Joshi, Salem Billan, Brett G. M. Hughes, Jean-Jacques Grob, Karthik Ramakrishnan, Joy Ge, Burak Gumuscu, Ramona F. Swaby, Ralf Gutzmer.
Corresponding author
Ethics declarations
Conflict of Interest
Åse Bratland received all support for the present manuscript from Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, personal and institutional payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Merck Sharp & Dohme and BMS and personal payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Sanofi, and is president of the Scandinavian Society of Clinical Oncology. Eva Munoz-Couselo received consulting fees from BMS, Merck Serono, Amgen, Pierre Fabre, Sanofi, and Roche, payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from BMS, Novartis, Sanofi, Merck Serono, and Pierre Fabre, support for attending meetings and/or travel from BMS, Novartis, Merck Serono, and Sun Pharma, and participates on a data safety monitoring board or advisory board for BMS. Laurent Mortier received support for attending meetings and/or travel from MSD, Merck, Novartis, BMS, and Pierre Fabre. Ana M. Arance received personal consulting fees from Pierre Fabre, Novartis, Roche, BMS, MSD, Sanofi, and Merck and personal payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Pierre Fabre, Novartis, Roche, BMS, MSD, Sanofi, and Merck. Florent Grange received consulting fees from Sanofi-Adventis, payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events and support for attending meetings and/or travel from BMS. Nicolas Meyer received institutional grants or contracts from BMS and MSD, personal consulting fees from BMS, MSD, Pierre Fabre, Merck, Sanofi, Sun Pharma, and Novartis, and support for attending meetings and/or travel from Pierre Fabre, MSD, and Novartis. Abhishek Jagdish Joshi received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from Ipsen, Janssen, and BMS, and support for attending meetings and/or travel from Ipsen, Roche, AstraZeneca, and Janssen. Brett G. M. Hughes received all support for the present manuscript from Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, institutional grants from Amgen, and payment or honoraria for speaker’s bureau from Eisai. Jean-Jacques Grob received personal consulting fees from BMS, Novartis, Amgen, Philogen, MSD, Pierre Fabre, Roche, and Sun Pharma, payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from BMA and Novartis, support for attending meetings and/or travel from Pierre Fabre, MSD, and BMS, participates on a data safety monitoring board or advisory board for BMS, MSD, Pierre Fabre, Novartis, and Philogen, and is in receipt of equipment, materials, drugs, medical writing, gifts, or other services from Pierre Fabre. Karthik Ramakrishnan is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Joy Ge has stock or stock options in Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Burak Gumuscu has stock in Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Ramona F. Swaby is a former employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, at the time of the conduct of the study; she reports current employment at Genmab US, 777 Scudders Mill Road, Bldg 2, Plainsboro, NJ 08536. Ralf Gutzmer received all support for the present manuscript from Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, consulting fees for participation on an advisory board from Roche, BMS, MSD, Novartis, Amgen, Merck Serono, Almirall Hermal, Sun Pharma, Sanofi, Pierre Fabre, Bayer, Pfizer, and Immunocore, consulting fees for participation on a data safety monitoring board from 4SC, honoraria for lectures from Roche, BMS, MSD, Novartis, Amgen, Merck Serono, Almirall Hermal, Sun Pharma, Sanofi, and Pierre Fabre, payment or honoraria for medical writing from BMS, Pfizer, Merck/MSD, Roche, Sun Pharma, Sanofi/Regeneron, Amgen, and Pierre Fabre, support for attending meetings and/or travel from Roche, BMS, Sun Pharma, Merck-Serono, and Pierre Fabre, and institutional support for investigator-initiated trials/projects from Novartis, Pfizer, Johnson & Johnson, Amgen, Merck-Serono, Sun Pharma, Sanofi, Kyowa Kirin, Almirall. Osama Roshdy, Rene González, Jacob Schachter, and Salem Billan have no conflicts of interest to disclose.
Ethical Approval
The study protocol and amendments were approved by the appropriate institutional review boards and ethics review committees at each institution (Supplementary Material Table 1). The study was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the Declaration of Helsinki. All participants provided written informed consent.
Additional information
Ramona F. Swaby: At the time the study was conducted.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bratland, Å., Munoz-Couselo, E., Mortier, L. et al. Health-Related Quality of Life with Pembrolizumab in Patients with Locally Advanced or Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: KEYNOTE-629. Dermatol Ther (Heidelb) 13, 3165–3180 (2023). https://doi.org/10.1007/s13555-023-01059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-01059-y