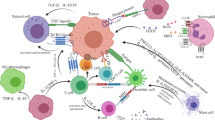
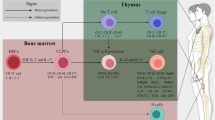
Abstract
Background
Natural killer (NK) cells have gained considerable attention and hold great potential for their application in tumor immunotherapy. This is mainly due to their MHC-unrestricted and pan-specific recognition capabilities, as well as their ability to rapidly respond to and eliminate target cells. To artificially generate therapeutic NK cells, various materials can be utilized, such as peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), induced pluripotent stem cells (iPSCs), and NK cell lines. Exploiting the therapeutic potential of NK cells to treat tumors through in vivo and in vitro therapeutic modalities has yielded positive therapeutic results.
Conclusion
This review provides a comprehensive description of NK cell therapeutic approaches for tumors and discusses the current problems associated with these therapeutic approaches and the prospects of NK cell therapy for tumors.


Similar content being viewed by others
Data availability
All data obtained and/or analyzed in this study were available from the corresponding author upon reasonable request.
Abbreviations
- 5T4:
-
Trophoblast glycoprotein
- ADCC:
-
Antibody-dependent cytotoxicity
- ALL:
-
Acute lymphoblastic leukemia
- AKT:
-
Protein kinase B
- AML:
-
Acute myeloid leukemia
- B7-H6:
-
B7 homolog 6
- BAT3:
-
HLA-B associated transcript 3
- BCMA:
-
B cell maturation antigen
- BiKE:
-
Bi-specific NK cell engagers
- C1q:
-
Complement component 1q
- CAR:
-
Chimeric antigen receptor
- CCL:
-
Chemokine (C-C motif) ligand
- CD:
-
Cluster of differentiation
- CEA:
-
Carcinoembryonic antigen
- CEACAM1:
-
Carcinoembryonic antigen-associated cell adhesion molecule-1
- CFP:
-
Complement factor P
- CLL:
-
Chronic lymphocytic leukemia
- CLL1:
-
C-type lectin-like molecule-1
- CIML NK:
-
Cytokine-induced memory-like NK cell
- CISH:
-
Cytokine-inducible SH2-containing protein
- CMV:
-
CytoMegalo Virus
- CR:
-
Complete response
- CRACC:
-
CD2-like receptor-activating cytotoxic cell
- CRS:
-
Cytokine release syndrome
- CRTAM:
-
Cytotoxic and regulatory T cell molecule
- CT26:
-
Colon tumor 26
- CTL:
-
Cytotoxic T lymphocyte
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DC:
-
Dendritic cell
- DLIs:
-
Donor lymphocyte infusions
- DLL3:
-
Delta-like ligand-3
- DNAM1:
-
DNAX accessory molecule-1
- DSGb5:
-
Disialosylglobopentaose
- EGFR:
-
Epidermal growth factor receptor
- Epa:
-
Epithelial adhesin
- ERK:
-
Extracellular signal-regulated kinase
- ESC:
-
Embryonic stem cell
- EV:
-
Extracellular Vesicle
- FcγRIIIA:
-
Fc gamma receptor IIIA
- FGL1:
-
Fibrinogen-like protein 1
- FOXO1:
-
Forkhead box protein O1
- GD:
-
Ganglioside
- GNLY:
-
Granulysin
- GzmA/B:
-
Granzyme A/B
- HA:
-
Hemagglutinin
- HCMV:
-
Human Cytomegalovirus
- HN:
-
Hemagglutinin-neuraminidase
- HNSCC:
-
Head and neck squamous cell carcinoma
- HSPG:
-
Heparan sulfate proteoglycans
- FasL:
-
Fas ligand
- FDA:
-
Food and drug administration
- FGL1:
-
Fibrinogen-like protein 1
- FLT3L:
-
FMS-like tyrosine kinase 3 receptor ligand
- Foxo1:
-
Forkhead box protein O1
- Gal9:
-
Galectin 9
- GBM:
-
Glioblastoma multiforme
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- GVHD:
-
Graft-versus-host disease
- haNK cell:
-
High-affinity NK cell
- HCC:
-
Hepatocellular carcinoma
- HCT:
-
Hematopoietic cell transplantation
- HER2:
-
Human epidermal growth factor receptor 2
- HIF:
-
Hypoxia-inducible factor
- HLA:
-
Human leukocyte antigen
- HMGB1:
-
High mobility group protein 1
- HNSCC:
-
Head and neck squamous cell carcinoma
- HSPCs:
-
Hematopoietic stem cells
- HSPG:
-
Heparan sulfate proteoglycan
- IgV:
-
Immunoglobulin variable region
- LAG3:
-
Lymphocyte activation gene 3
- LAIR-1:
-
Leucocyte-associated immunoglobulin-like receptor-1
- LIRs:
-
Leukocyte immunoglobulin-like receptors
- LLT-1:
-
Lectin-like transcript 1
- LSECtin:
-
Liver sinusoidal endothelial cell lectin
- ICI:
-
Immune checkpoint inhibitor
- IFN:
-
Interferon
- IL:
-
Interleukin
- ILT2:
-
Ig-like transcript 2
- IRp60:
-
Inhibitory receptor protein 60
- iPSCs:
-
Induced Pluripotent stem cells
- irAEs:
-
Immune-related adverse events
- ITIM:
-
Immunoreceptor tyrosine inhibitory motif
- JAK:
-
Janus kinase
- KIR:
-
The killer cell immunoglobulin-like receptor
- KLRG1:
-
Co-inhibitory receptor killer-cell lectin like receptor G1
- LAG-3:
-
Lymphocyte activation gene-3
- mAb:
-
Monoclonal Antibody
- mAGP:
-
Mycolyl-arabinogalactan-peptidoglycan
- MAPK:
-
Mitogen-activated protein kinase
- MDS:
-
Myelodysplastic syndromes
- MDSC:
-
Myeloid-derived suppressor cell
- METTL3:
-
Methyltransferase-like 3
- MICA:
-
MHC class I polypeptide-related sequence A
- MICB:
-
MHC class I polypeptide-related sequence B
- MHC:
-
Major histocompatibility complex
- MLL5:
-
Mixed lineage leukemia 5
- MM:
-
Multiple myeloma
- MSLN:
-
Mesothelin
- MUC:
-
Mucin
- mTOR:
-
Mammalian target of rapamycin
- NCRs:
-
Natural cytotoxicity receptors
- NECL2:
-
Nectin-like protein 2
- NEJM:
-
New England Journal of Medicine
- NETs:
-
Neutrophil extracellular traps
- NHL:
-
Non-Hodgkin lymphoma
- NID1:
-
Nidogen-1
- NKCE:
-
NK cell engagers
- NK cell:
-
Natural killer cell
- NKG2:
-
Nature-killer group 2
- NKP:
-
NK precursor cells
- NKp30/44/46:
-
Natural cytotoxicity receptor 30/44/46
- NKRP1A:
-
Natural killer cell receptor-P1A
- NSCLC:
-
Non-small cell lung cancer
- NTB-A:
-
NK-T-B-antigen
- oHSV:
-
Oncolytic herpes simplex virus
- OV:
-
Oncolytic virus
- PBMCs:
-
Peripheral blood mononuclear cells
- PCNA:
-
Proliferating cell nuclear antigen
- PD-1:
-
Programmed cell death protein 1
- PDGF:
-
Platelet-derived growth factor
- PE:
-
Phosphatidylethanolamine
- PfEMP-1:
-
Plasmodium falciparum erythrocyte membrane protein 1
- PFN:
-
Perforin
- PI3K:
-
Phosphoinositide 3-kinase
- PGE2:
-
Prostaglandin E2
- PS:
-
Phosphatidylserine
- PSCA:
-
Prostate stem cell antigen
- PSMA:
-
Prostate specific membrane antigen
- Ptdser:
-
Phospholipid serine
- PVR:
-
Poliovirus receptor
- PVRL2:
-
Poliovirus receptor related2
- rhIL-15:
-
Recombinant human interleukin-15
- ROBO1:
-
Roundabout guidance receptor 1
- SCF:
-
Stem cell factor
- scFv:
-
Single-chain variable fragment
- SHP:
-
Src homology region 2 domain-containing phosphatase
- Siglec:
-
Sialic acid-binding immunoglobulin-like lectins
- siRNA:
-
Small interfering RNA
- STAT1:
-
Signal transducer and activator of transcription 1
- TCRs:
-
T-cell receptors
- TIGIT:
-
T-cell immunoreceptor with Ig and ITIM domains
- TIM-3:
-
T cell immunoglobulin domain and mucin domain-containing protein 3
- TME:
-
Tumor microenvironment
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
Tumor necrosis factor-associated apoptosis-inducing ligand
- TriKE:
-
Tri-specific NK cell engagers
- UCB:
-
Umbilical cord blood
- ULBP:
-
UL16-binding protein
- VAP-1:
-
Vascular adhesion protein-
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
D. Hanahan, Hallmarks of Cancer: New dimensions. Cancer Discov. 12, 31–46 (2022)
A.K. Singh, J.P. McGuirk, CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 21, e168–e178 (2020)
S. Bagchi, R. Yuan, E.G. Engleman, Immune Checkpoint inhibitors for the treatment of Cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021)
O. Demaria, S. Cornen, M. Daëron, Y. Morel, R. Medzhitov, E. Vivier, Harnessing innate immunity in cancer therapy. Nature. 574, 45–56 (2019)
Y. Yang, Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 125, 3335–3337 (2015)
A. Kumar, C.A. Swain, L.A. Shevde, Informing the new developments and future of cancer immunotherapy. Cancer Metastasis Rev. 40, 549–562 (2021)
M.G. Morvan, L.L. Lanier, NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer. 16, 7–19 (2016)
T. Timonen, J.R. Ortaldo, R.B. Herberman, Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J. Exp. Med. 153, 569–582 (1981)
M. Anft, P. Netter, D. Urlaub, I. Prager, S. Schaffner, C. Watzl, NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell. Mol. Immunol. 17, 347–355 (2020)
F. Fang, S. Xie, M. Chen, Y. Li, J. Yue, J. Ma et al., Advances in NK cell production. Cell. Mol. Immunol. 19, 460–481 (2022)
M.A. Caligiuri, Human natural killer cells. Blood. 112, 461–469 (2008)
C. Fauriat, E.O. Long, H.-G. Ljunggren, Y.T. Bryceson, Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 115, 2167–2176 (2010)
J. Yu, A.G. Freud, M.A. Caligiuri, Location and cellular stages of NK cell development. Trends Immunol. 34 (2013). https://doi.org/10.1016/j.it.2013.07.005
P. Dogra, C. Rancan, W. Ma, M. Toth, T. Senda, D.J. Carpenter et al., Tissue determinants of human NK Cell Development, function, and Residence. Cell. 180, 749–763e13 (2020)
E. Vivier, D.H. Raulet, A. Moretta, M.A. Caligiuri, L. Zitvogel, L.L. Lanier et al., Innate or adaptive immunity? The Example of Natural Killer cells. Science. 331, 44–49 (2011)
S. Kumar, Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology. 154, 383–393 (2018)
F. Nimmerjahn, J.V. Ravetch, Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008)
J.S. Orange, Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 8, 713–725 (2008)
I. Prager, C. Watzl, Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 105, 1319–1329 (2019)
M.J. Smyth, E. Cretney, J.M. Kelly, J.A. Westwood, S.E.A. Street, H. Yagita et al., Activation of NK cell cytotoxicity. Mol. Immunol. 42, 501–510 (2005)
I. Prager, C. Liesche, van H. Ooijen, D. Urlaub, Q. Verron, N. Sandström et al., NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 216, 2113–2127 (2019)
D. Piccioli, S. Sbrana, E. Melandri, N.M. Valiante, Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002)
B. Cózar, M. Greppi, S. Carpentier, E. Narni-Mancinelli, L. Chiossone, E. Vivier, Tumor-infiltrating natural killer cells. Cancer Discov. 11, 34–44 (2021)
E. Schlecker, N. Fiegler, A. Arnold, P. Altevogt, S. Rose-John, G. Moldenhauer et al., Metalloprotease-mediated Tumor Cell Shedding of B7-H6, the ligand of the natural killer cell–activating receptor NKp30. Cancer Res. 74, 3429–3440 (2014)
F. Ghiringhelli, C. Ménard, M. Terme, C. Flament, J. Taieb, N. Chaput et al., CD4 + CD25 + regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 202, 1075–1085 (2005)
Á. Teijeira, S. Garasa, M. Gato, C. Alfaro, I. Migueliz, A. Cirella et al., CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce Neutrophil Extracellular traps that interfere with Immune cytotoxicity. Immunity. 52, 856–871e8 (2020)
X. Zheng, Y. Qian, B. Fu, D. Jiao, Y. Jiang, P. Chen et al., Mitochondrial fragmentation limits NK cell-based Tumor immunosurveillance. Nat. Immunol. 20, 1656–1667 (2019)
J. Ni, X. Wang, A. Stojanovic, Q. Zhang, M. Wincher, L. Bühler et al., Single-cell RNA sequencing of Tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK Cell activity. Immunity. 52, 1075–1087e8 (2020)
S.E. Keating, V. Zaiatz-Bittencourt, R.M. Loftus, C. Keane, K. Brennan, D.K. Finlay et al., Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J. Immunol. 196, 2552–2560 (2016)
M.G. Badur, C.M. Metallo, Reverse engineering the cancer metabolic network using flux analysis to understand drivers of human Disease. Metab. Eng. 45, 95–108 (2018)
C.-H. Chang, J. Qiu, D. O’Sullivan, M.D. Buck, T. Noguchi, J.D. Curtis et al., Metabolic competition in the Tumor Microenvironment is a driver of Cancer Progression. Cell. 162, 1229–1241 (2015)
R.M. Loftus, N. Assmann, N. Kedia-Mehta, K.L. O’Brien, A. Garcia, C. Gillespie et al., Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 9, 2341 (2018)
X. Zheng, Z. Hou, Y. Qian, Y. Zhang, Q. Cui, X. Wang et al., Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat. Immunol. 24, 802–813 (2023)
X. Michelet, L. Dyck, A. Hogan, R.M. Loftus, D. Duquette, K. Wei et al., Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018)
C. Harmon, M.W. Robinson, F. Hand, D. Almuaili, K. Mentor, D.D. Houlihan et al., Lactate-mediated acidification of Tumor Microenvironment induces apoptosis of Liver-Resident NK cells in Colorectal Liver Metastasis. Cancer Immunol. Res. 7, 335–346 (2019)
D.J. Propper, F.R. Balkwill, Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022)
N.W. Zwirner, C.I. Domaica, Cytokine regulation of natural killer cell effector functions. BioFactors. 36, 274–288 (2010)
R. Spolski, P. Li, W.J. Leonard, Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat. Rev. Immunol. 18, 648–659 (2018)
W. Domzig, B.M. Stadler, R.B. Herberman, Interleukin 2 dependence of human natural killer (NK) cell activity. J. Immunol. 130, 1970–1973 (1983)
J.P. Siegel, R.K. Puri, Interleukin-2 toxicity. J. Clin. Oncol. 9, 694–704 (1991)
K.E. Harris, K.J. Lorentsen, H.K. Malik-Chaudhry, K. Loughlin, H.M. Basappa, S. Hartstein et al., A bispecific antibody agonist of the IL-2 heterodimeric receptor preferentially promotes in vivo expansion of CD8 and NK cells. Sci. Rep. 11, 10592 (2021)
A.M. Levin, D.L. Bates, A.M. Ring, C. Krieg, J.T. Lin, L. Su et al., Exploiting a natural conformational switch to engineer an Interleukin-2 superkine. Nature. 484, 529–533 (2012)
N. Arenas-Ramirez, C. Zou, S. Popp, D. Zingg, B. Brannetti, E. Wirth et al., Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci. Transl Med. 8, 367ra166 (2016)
J.L. Ptacin, C.E. Caffaro, L. Ma, K.M. San Jose Gall, H.R. Aerni, N.V. Acuff et al., An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat. Commun. 12, 4785 (2021)
B. Becknell, M.A. Caligiuri, Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 86, 209–239 (2005)
J.G. Giri, D.M. Anderson, S. Kumaki, L.S. Park, K.H. Grabstein, D. Cosman, IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J. Leukoc. Biol. 57, 763–766 (1995)
A.K. Ali, N. Nandagopal, S.-H. Lee, IL-15-PI3K-AKT-mTOR: a critical pathway in the Life Journey of Natural Killer cells. Front. Immunol. 6, 355 (2015)
X. Zhou, J. Yu, X. Cheng, B. Zhao, G.C. Manyam, L. Zhang et al., The deubiquitinase Otub1 controls the activation of CD8 + T cells and NK cells by regulating IL-15-mediated priming. Nat. Immunol. 20, 879–889 (2019)
Y. Wang, Y. Zhang, P. Yi, W. Dong, A.P. Nalin, J. Zhang et al., The IL-15-AKT-XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat. Immunol. 20, 10–17 (2019)
K. Imada, E.T. Bloom, H. Nakajima, J.A. Horvath-Arcidiacono, G.B. Udy, H.W. Davey et al., Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J. Exp. Med. 188, 2067–2074 (1998)
H. Song, J. Song, M. Cheng, M. Zheng, T. Wang, S. Tian et al., METTL3-mediated m6A RNA methylation promotes the anti-tumour immunity of natural killer cells. Nat. Commun. 12, 5522 (2021)
M. Zhang, B. Wen, O.M. Anton, Z. Yao, S. Dubois, W. Ju et al., IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl. Acad. Sci. U S A 115, E10915–E10924 (2018)
Y. Guo, L. Luan, N.K. Patil, E.R. Sherwood, Immunobiology of the IL-15-IL-15Rα complex as an Antitumor and Antiviral Agent. Cytokine Growth Factor Rev. 38, 10–21 (2017)
S. Zhang, J. Zhao, X. Bai, M. Handley, F. Shan, Biological effects of IL-15 on immune cells and its potential for the treatment of cancer. Int. Immunopharmacol. 91, 107318 (2021)
S. Cooley, F. He, V. Bachanova, G.M. Vercellotti, T.E. DeFor, J.M. Curtsinger et al., First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute Myeloid Leukemia. Blood Adv. 3, 1970–1980 (2019)
K.M. Knudson, J.W. Hodge, J. Schlom, S.R. Gameiro, Rationale for IL-15 superagonists in cancer immunotherapy. Expert Opin. Biol. Ther. 20, 705–709 (2020)
K. Margolin, C. Morishima, V. Velcheti, J.S. Miller, S.M. Lee, A.W. Silk et al., Phase I Trial of ALT-803, a novel recombinant IL15 complex, in patients with Advanced Solid tumors. Clin. Cancer Res. 24, 5552–5561 (2018)
J.M. Wrangle, V. Velcheti, M.R. Patel, E. Garrett-Mayer, E.G. Hill, J.G. Ravenel et al., ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell Lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018)
J.A. Hangasky, W. Chen, S.P. Dubois, A. Daenthanasanmak, J.R. Müller, R. Reid et al., A very long-acting IL-15: implications for the immunotherapy of cancer. J. Immunother Cancer. 10, e004104 (2022)
M. Patidar, N. Yadav, S.K. Dalai, Interleukin 15: a key cytokine for immunotherapy. Cytokine Growth Factor Rev. 31, 49–59 (2016)
S.A. Perez, L.G. Mahaira, P.A. Sotiropoulou, A.D. Gritzapis, E.G. Iliopoulou, D.K. Niarchos et al., Effect of IL-21 on NK cells derived from different umbilical cord blood populations. Int. Immunol. 18, 49–58 (2006)
J. Parrish-Novak, S.R. Dillon, A. Nelson, A. Hammond, C. Sprecher, J.A. Gross et al., Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 408, 57–63 (2000)
K. Skak, K.S. Frederiksen, D. Lundsgaard, Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 123, 575–583 (2008)
H. Seo, I. Jeon, B.-S. Kim, M. Park, E.-A. Bae, B. Song et al., IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 8, 15776 (2017)
M. Khan, S. Arooj, H. Wang, NK Cell-based Immune Checkpoint Inhibition. Front. Immunol. 11, 167 (2020)
G. Morad, B.A. Helmink, P. Sharma, J.A. Wargo, Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 184, 5309–5337 (2021)
N. Stanietsky, H. Simic, J. Arapovic, A. Toporik, O. Levy, A. Novik et al., The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. U S A 106, 17858–17863 (2009)
K.B. Lupo, S. Matosevic, CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J. Hematol. Oncol. 13, 76 (2020)
Q. Zhang, J. Bi, X. Zheng, Y. Chen, H. Wang, W. Wu et al., Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018)
G. Liu, Q. Zhang, J. Yang, X. Li, L. Xian, W. Li et al., Increased TIGIT expressing NK cells with dysfunctional phenotype in AML patients correlated with poor prognosis. Cancer Immunol. Immunother. 71, 277–287 (2022)
L. Martinet, M.J. Smyth, Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 15, 243–254 (2015)
B.C. Cho, D.R. Abreu, M. Hussein, M. Cobo, A.J. Patel, N. Secen et al., Tiragolumab plus Atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell Lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23, 781–792 (2022)
Tiragolumab Results Cast Shadow on TIGIT Pipeline, Cancer Discov. 12, 1603–1604 (2022)
F. Borrego, M. Masilamani, J. Kabat, T.B. Sanni, J.E. Coligan, The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol. Immunol. 42, 485–488 (2005)
L. Borst, van der S.H. Burg, van T. Hall, The NKG2A–HLA-E Axis as a Novel checkpoint in the Tumor Microenvironment. Clin. Cancer Res. 26, 5549–5556 (2020)
C. Sun, J. Xu, Q. Huang, M. Huang, H. Wen, C. Zhang et al., High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with Liver cancer. Oncoimmunology. 6, e1264562 (2016)
E.M. McWilliams, J.M. Mele, C. Cheney, E.A. Timmerman, F. Fiazuddin, E.J. Strattan et al., Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic Leukemia. Oncoimmunology. 5, e1226720 (2016)
P. André, C. Denis, C. Soulas, C. Bourbon-Caillet, J. Lopez, T. Arnoux et al., Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK Cells. Cell. 175, 1731–1743e13 (2018)
A.V. Tinker, H.W. Hirte, D. Provencher, M. Butler, H. Ritter, D. Tu et al., Dose-ranging and cohort-expansion study of Monalizumab (IPH2201) in patients with Advanced Gynecologic malignancies: a trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 25, 6052–6060 (2019)
N.H. Segal, J. Naidoo, G. Curigliano, S. Patel, S. Sahebjam, K.P. Papadopoulos et al., First-in-human dose escalation of monalizumab plus durvalumab, with expansion in patients with metastatic microsatellite-stable Colorectal cancer. JCO. 36, 3540–3540 (2018)
C. Zhu, A.C. Anderson, A. Schubart, H. Xiong, J. Imitola, S.J. Khoury et al., The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005)
Y. Wolf, A.C. Anderson, V.K. Kuchroo, TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 20, 173–185 (2020)
R. Yang, L. Sun, C.-F. Li, Y.-H. Wang, J. Yao, H. Li et al., Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 12, 832 (2021)
M. Das, C. Zhu, V.K. Kuchroo, Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111 (2017)
L.C. Ndhlovu, S. Lopez-Vergès, J.D. Barbour, R.B. Jones, A.R. Jha, B.R. Long et al., Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 119, 3734–3743 (2012)
da I.P. Silva, A. Gallois, S. Jimenez-Baranda, S. Khan, A.C. Anderson, V.K. Kuchroo et al., Reversal of NK cell exhaustion in advanced Melanoma by Tim-3 blockade. Cancer Immunol. Res. 2, 410–422 (2014)
L. Xu, Y. Huang, L. Tan, W. Yu, D. Chen, C. Lu et al., Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 29, 635–641 (2015)
S. Tan, Y. Xu, Z. Wang, T. Wang, X. Du, X. Song et al., Tim-3 hampers Tumor Surveillance of Liver-Resident and Conventional NK cells by disrupting PI3K signaling. Cancer Res. 80, 1130–1142 (2020)
O. Demaria, L. Gauthier, G. Debroas, E. Vivier, Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur. J. Immunol. 51, 1934–1942 (2021)
L.N. Kerbauy, N.D. Marin, M. Kaplan, P.P. Banerjee, M.M. Berrien-Elliott, M. Becker-Hapak et al., Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30 + malignancies. Clin. Cancer Res. 27, 3744–3756 (2021)
A. Rothe, S. Sasse, M.S. Topp, D.A. Eichenauer, H. Hummel, K.S. Reiners et al., A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin Lymphoma. Blood. 125, 4024–4031 (2015)
N.L. Bartlett, A.F. Herrera, E. Domingo-Domenech, A. Mehta, A. Forero-Torres, R. Garcia-Sanz et al., A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin Lymphoma. Blood. 136, 2401–2409 (2020)
C. Zhang, J. Röder, A. Scherer, M. Bodden, J. Pfeifer Serrahima, A. Bhatti et al., Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J. Immunother Cancer. 9, e002980 (2021)
Y. Wang, H. Li, W. Xu, M. Pan, C. Qiao, J. Cai et al., BCMA-targeting bispecific antibody that simultaneously stimulates NKG2D-enhanced efficacy against Multiple Myeloma. J. Immunother. 43, 175–188 (2020)
T. Wang, F. Sun, W. Xie, M. Tang, H. He, X. Jia et al., A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett. 372, 166–178 (2016)
von E.P. Strandmann, H.P. Hansen, K.S. Reiners, R. Schnell, P. Borchmann, S. Merkert et al., A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human Multiple Myeloma in vitro and in vivo. Blood. 107, 1955–1962 (2006)
T. Wang, F. Sun, Y. Wang, J. Jiang, M. Pan, M. Yuan et al., NKG2D Immunoligand rG7S-MICA enhances NK Cell-mediated Immunosurveillance in Colorectal Carcinoma. J. Immunother. 41, 109–117 (2018)
D.A. Vallera, M. Felices, R. McElmurry, V. McCullar, X. Zhou, J.U. Schmohl et al., IL-15 trispecific killer engagers (TriKEs) make natural killer cells specific to CD33 + targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res. 22, 3440–3450 (2016)
M. Felices, T.R. Lenvik, B. Kodal, A.J. Lenvik, P. Hinderlie, L.E. Bendzick et al., Potent cytolytic activity and specific IL15 delivery in a second-generation Trispecific Killer Engager. Cancer Immunol. Res. 8, 1139–1149 (2020)
L. Gauthier, A. Morel, N. Anceriz, B. Rossi, A. Blanchard-Alvarez, G. Grondin et al., Multifunctional natural killer cell engagers targeting NKp46 trigger protective Tumor immunity. Cell. 177, 1701–1713e16 (2019)
L. Gauthier, A. Virone-Oddos, J. Beninga, B. Rossi, C. Nicolazzi, C. Amara et al., Control of acute Myeloid Leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat. Biotechnol. 41, 1296–1306 (2023)
O. Demaria, L. Gauthier, M. Vetizou, A. Blanchard Alvarez, C. Vagne, G. Habif et al., Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell. Rep. Med. 3, 100783 (2022)
M. Marotel, M.S. Hasim, A. Hagerman, M. Ardolino, The two-faces of NK cells in oncolytic virotherapy. Cytokine Growth Factor Rev. 56, 59–68 (2020)
M. Wantoch, E.B. Wilson, A.P. Droop, S.L. Phillips, M. Coffey, Y.M. El-Sherbiny et al., Oncolytic virus treatment differentially affects the CD56dim and CD56bright NK cell subsets in vivo and regulates a spectrum of human NK cell activity. Immunology. 166, 104–120 (2022)
C.A. Alvarez-Breckenridge, J. Yu, R. Price, J. Wojton, J. Pradarelli, H. Mao et al., NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat. Med. 18, 1827–1834 (2012)
B. Xu, R. Ma, L. Russell, J.Y. Yoo, J. Han, H. Cui et al., An oncolytic herpes virus expressing E-cadherin resists NK cell clearance and improves viral spread and glioblastoma virotherapy. Nat Biotechnol. 2018;10.1038/nbt.4302
A.P. Aspirin, de V.A.A. Los Reyes, Y. Kim, Polytherapeutic strategies with oncolytic virus-bortezomib and adjuvant NK cells in cancer treatment. J. R Soc. Interface. 18, 20200669 (2021)
N.S. Senekal, K.J. Mahasa, A. Eladdadi, de L. Pillis, R. Ouifki, Natural Killer Cells Recruitment in Oncolytic Virotherapy: a Mathematical Model. Bull. Math. Biol. 83, 75 (2021)
Y. Kim, J.Y. Yoo, T.J. Lee, J. Liu, J. Yu, M.A. Caligiuri et al., Complex role of NK cells in regulation of oncolytic virus-bortezomib therapy. Proc. Natl. Acad. Sci. U S A 115, 4927–4932 (2018)
A.A. Alkayyal, L.-H. Tai, M.A. Kennedy, de C.T. Souza, J. Zhang, C. Lefebvre et al., NK-Cell recruitment is necessary for eradication of peritoneal carcinomatosis with an IL12-Expressing Maraba Virus Cellular Vaccine. Cancer Immunol. Res. 5, 211–221 (2017)
K. Rajani, C. Parrish, T. Kottke, J. Thompson, S. Zaidi, L. Ilett et al., Combination therapy with Reovirus and Anti-PD-1 Blockade controls Tumor Growth through Innate and Adaptive Immune responses. Mol. Ther. 24, 166–174 (2016)
S.B. Willingham, J.-P. Volkmer, A.J. Gentles, D. Sahoo, P. Dalerba, S.S. Mitra et al., The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U S A 109, 6662–6667 (2012)
L. Tian, B. Xu, K.-Y. Teng, M. Song, Z. Zhu, Y. Chen et al., Targeting fc receptor-mediated effects and the don’t eat me signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic Ovarian cancer. Clin. Cancer Res. 28, 201–214 (2022)
A.M. Noonan, M.R. Farren, S.M. Geyer, Y. Huang, S. Tahiri, D. Ahn et al., Randomized phase 2 trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 24, 1150–1158 (2016)
N. Lamers-Kok, D. Panella, A.-M. Georgoudaki, H. Liu, D. Özkazanc, L. Kučerová et al., Natural killer cells in clinical development as non-engineered, engineered, and combination therapies. J. Hematol. Oncol. 15, 164 (2022)
T. Mamo, S.M. Williams, S. Kinney, K.M. Tessier, T.E. DeFor, S. Cooley et al., Infusion reactions in natural killer cell immunotherapy: a retrospective review. Cytotherapy. 23, 627–634 (2021)
A. Curti, L. Ruggeri, S. Parisi, A. Bontadini, E. Dan, M.R. Motta et al., Larger size of Donor Alloreactive NK Cell Repertoire correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia patients. Clin. Cancer Res. 22, 1914–1921 (2016)
S. Parisi, L. Ruggeri, E. Dan, S. Rizzi, B. Sinigaglia, D. Ocadlikova et al., Long-term Outcome after adoptive Immunotherapy with Natural Killer cells: alloreactive NK cell dose still matters. Front. Immunol. 12, 804988 (2022)
J.H. Gong, G. Maki, H.G. Klingemann, Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 8, 652–658 (1994)
G. Maki, H.G. Klingemann, J.A. Martinson, Y.K. Tam, Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J. Hematother Stem Cell. Res. 10, 369–383 (2001)
H. Klingemann, L. Boissel, F. Toneguzzo, Natural killer cells for immunotherapy – advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 7, 91 (2016)
S. Nagashima, R. Mailliard, Y. Kashii, T.E. Reichert, R.B. Herberman, P. Robbins et al., Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood. 91, 3850–3861 (1998)
Y.K. Tam, G. Maki, B. Miyagawa, B. Hennemann, T. Tonn, H.G. Klingemann, Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 10, 1359–1373 (1999)
L. Gao, L. Yang, S. Zhang, Z. Ge, M. Su, Y. Shi et al., Engineering NK-92 cell by upregulating CXCR2 and IL-2 Via CRISPR-Cas9 improves its Antitumor effects as Cellular Immunotherapy for human Colon Cancer. J. Interferon Cytokine Res. 41, 450–460 (2021)
C. Jochems, J.W. Hodge, M. Fantini, R. Fujii, Y.M. Maurice, J.W. Greiner et al., An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 7, 86359–86373 (2016)
J. Zhang, R. Sun, H. Wei, J. Zhang, Z. Tian, Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 89, 338–347 (2004)
S. Arai, R. Meagher, M. Swearingen, H. Myint, E. Rich, J. Martinson et al., Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or Melanoma: a phase I trial. Cytotherapy. 10, 625–632 (2008)
T. Tonn, D. Schwabe, H.G. Klingemann, S. Becker, R. Esser, U. Koehl et al., Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 15, 1563–1570 (2013)
M. Boyiadzis, M. Agha, R.L. Redner, A. Sehgal, A. Im, J.-Z. Hou et al., Phase 1 clinical trial of adoptive immunotherapy using off-the-shelf activated natural killer cells in patients with refractory and relapsed acute Myeloid Leukemia. Cytotherapy. 19, 1225–1232 (2017)
S.A. Lim, T.-J. Kim, J.E. Lee, C.H. Sonn, K. Kim, J. Kim et al., Ex vivo expansion of highly cytotoxic human NK cells by cocultivation with irradiated Tumor cells for adoptive immunotherapy. Cancer Res. 73, 2598–2607 (2013)
Y.H. Choi, E.J. Lim, S.W. Kim, Y.W. Moon, K.S. Park, H.-J. An, IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J. Immunother Cancer. 7, 168 (2019)
C.J. Denman, V.V. Senyukov, S.S. Somanchi, P.V. Phatarpekar, L.M. Kopp, J.L. Johnson et al., Membrane-bound IL-21 promotes sustained Ex vivo proliferation of human natural killer cells. PLoS One. 7, e30264 (2012)
X.-Y. Zhao, Q. Jiang, H. Jiang, L.-J. Hu, T. Zhao, X.-X. Yu et al., Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute Myeloid Leukemia in vivo. Eur. J. Immunol. 50, 1374–1385 (2020)
S.O. Ciurea, J.R. Schafer, R. Bassett, C.J. Denman, K. Cao, D. Willis et al., Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood. 130, 1857–1868 (2017)
S.O. Ciurea, P. Kongtim, D. Soebbing, P. Trikha, G. Behbehani, G. Rondon et al., Decrease post-transplant relapse using donor-derived expanded NK-cells. Leukemia. 36, 155–164 (2022)
J.L. Oyer, V. Pandey, R.Y. Igarashi, S.S. Somanchi, A. Zakari, M. Solh et al., Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 18, 653–663 (2016)
J.L. Oyer, T.J. Croom-Perez, T.A. Dieffenthaller, L.D. Robles-Carillo, S.B. Gitto, D.A. Altomare et al., Cryopreserved PM21-Particle-expanded natural killer cells maintain cytotoxicity and Effector functions in Vitro and in vivo. Front. Immunol. 13, 861681 (2022)
J.S. Miller, Y. Soignier, A. Panoskaltsis-Mortari, S.A. McNearney, G.H. Yun, S.K. Fautsch et al., Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 105, 3051–3057 (2005)
L. Ruggeri, M. Capanni, E. Urbani, K. Perruccio, W.D. Shlomchik, A. Tosti et al., Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 295, 2097–2100 (2002)
J.E. Rubnitz, H. Inaba, R.C. Ribeiro, S. Pounds, B. Rooney, T. Bell et al., NKAML: a pilot study to determine the safety and feasibility of Haploidentical Natural Killer Cell Transplantation in Childhood Acute Myeloid Leukemia. J. Clin. Oncol. 28, 955–959 (2010)
A. Curti, L. Ruggeri, A. D’Addio, A. Bontadini, E. Dan, M.R. Motta et al., Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute Myeloid Leukemia patients. Blood. 118, 3273–3279 (2011)
W. Hu, G. Wang, D. Huang, M. Sui, Y. Xu, Cancer Immunotherapy based on natural killer cells: current progress and New opportunities. Front. Immunol. 10, 1205 (2019)
M. Lin, H. Luo, S. Liang, J. Chen, A. Liu, L. Niu et al., Pembrolizumab plus allogeneic NK cells in advanced non-small cell Lung cancer patients. J. Clin. Invest. 130, 2560–2569 (2020)
S. Querol, P. Rubinstein, A. Madrigal, The wider perspective: cord blood banks and their future prospects. Br. J. Haematol. 195, 507–517 (2021)
T. Nham, S.M. Poznanski, I.Y. Fan, F. Vahedi, M.M. Shenouda, A.J. Lee et al., Ex vivo-expanded natural killer cells derived from long-term Cryopreserved Cord blood are cytotoxic against primary Breast Cancer cells. J. Immunother. 41, 64–72 (2018)
R. Alnabhan, A. Madrigal, A. Saudemont, Differential activation of cord blood and peripheral blood natural killer cells by cytokines. Cytotherapy. 17, 73–85 (2015)
C. Reina-Ortiz, M. Constantinides, A. Fayd-Herbe-de-Maudave, J. Présumey, J. Hernandez, G. Cartron et al., Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against Tumor cells from Multiple Myeloma patients. OncoImmunology. 10, 1853314 (2021)
L. Herrera, J.M. Salcedo, S. Santos, M. Vesga, F. Borrego, C. Eguizabal, OP9 feeder cells are Superior to M2-10B4 cells for the generation of mature and functional natural killer cells from umbilical cord hematopoietic progenitors. Front. Immunol. 8, 755 (2017)
J. Spanholtz, M. Tordoir, D. Eissens, F. Preijers, van der A. Meer, I. Joosten et al., High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One. 5, e9221 (2010)
H. Dolstra, M.W.H. Roeven, J. Spanholtz, B.N. Hangalapura, M. Tordoir, F. Maas et al., Successful transfer of umbilical cord blood CD34 + hematopoietic stem and progenitor-derived NK Cells in older Acute Myeloid Leukemia patients. Clin. Cancer Res. 23, 4107–4118 (2017)
D.A. Knorr, Z. Ni, D. Hermanson, M.K. Hexum, L. Bendzick, L.J.N. Cooper et al., Clinical-scale derivation of natural killer cells from human pluripotent stem cells for Cancer Therapy. Stem Cells Transl Med. 2, 274–283 (2013)
H. Zhu, D.S. Kaufman, An Improved Method to produce clinical-scale natural killer cells from human pluripotent stem cells. Methods Mol. Biol. 2048, 107–119 (2019)
K.B. Lupo, J.-I. Moon, A.M. Chambers, S. Matosevic, Differentiation of natural killer cells from induced pluripotent stem cells under defined, serum- and feeder-free conditions. Cytotherapy. 23, 939–952 (2021)
K. Shankar, C.M. Capitini, K. Saha, Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell. Res. Ther. 11, 234 (2020)
F. Cichocki, R. Bjordahl, S. Gaidarova, S. Mahmood, R. Abujarour, H. Wang et al., iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo Tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl Med. 12, eaaz5618 (2020)
P.S. Woll, B. Grzywacz, X. Tian, R.K. Marcus, D.A. Knorr, M.R. Verneris et al., Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 113, 6094–6101 (2009)
D.L. Hermanson, L. Bendzick, L. Pribyl, V. McCullar, R.I. Vogel, J.S. Miller et al., Induced pluripotent stem cell-derived natural killer cells for treatment of Ovarian cancer. Stem Cells. 34, 93–101 (2016)
M.M. Berrien-Elliott, M.T. Jacobs, T.A. Fehniger, Allogeneic natural killer cell therapy. Blood. 141, 856 (2023)
H. Zhu, R.H. Blum, R. Bjordahl, S. Gaidarova, P. Rogers, T.T. Lee et al., Pluripotent stem cell–derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 135, 399–410 (2020)
H. Zhu, R.H. Blum, D. Bernareggi, E.H. Ask, Z. Wu, H.J. Hoel et al., Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell. Stem Cell. 27, 224–237e6 (2020)
H. Dong, J.D. Ham, G. Hu, G. Xie, J. Vergara, Y. Liang et al., Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute Myeloid Leukemia. Proc. Natl. Acad. Sci. U S A 119, e2122379119 (2022)
J.C. Sun, L.L. Lanier, Is there natural killer cell memory and can it be harnessed by vaccination? Cold Spring Harb Perspect Biol. 10, a029538 (2018)
A. Cerwenka, L.L. Lanier, Natural killer cell memory in Infection, inflammation and cancer. Nat. Rev. Immunol. 16, 112–123 (2016)
I. Terrén, A. Orrantia, A. Mosteiro, J. Vitallé, O. Zenarruzabeitia, F. Borrego, Metabolic changes of Interleukin-12/15/18-stimulated human NK cells. Sci. Rep. 11, 6472 (2021)
E.-M. Ewen, J.H.W. Pahl, M. Miller, C. Watzl, A. Cerwenka, KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of Tumor cells. Eur. J. Immunol. 48, 355–365 (2018)
J.W. Leong, J.M. Chase, R. Romee, S.E. Schneider, R.P. Sullivan, M.A. Cooper et al., Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant. 20, 463–473 (2014)
M. Boieri, A. Ulvmoen, A. Sudworth, C. Lendrem, M. Collin, A.M. Dickinson et al., IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic Leukemia and delay Leukemia development in vivo. OncoImmunology. 6, e1274478 (2017)
M.A. Cooper, J.M. Elliott, P.A. Keyel, L. Yang, J.A. Carrero, W.M. Yokoyama, Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U S A 106, 1915–1919 (2009)
J. Ni, M. Miller, A. Stojanovic, N. Garbi, A. Cerwenka, Sustained effector function of IL-12/15/18–preactivated NK cells against established tumors. J. Exp. Med. 209, 2351–2365 (2012)
R. Romee, M. Rosario, M.M. Berrien-Elliott, J.A. Wagner, B.A. Jewell, T. Schappe et al., Cytokine-induced memory-like natural killer cells exhibit enhanced responses against Myeloid Leukemia. Sci. Transl Med. 8, 357ra123 (2016)
N.D. Marin, B.A. Krasnick, M. Becker-Hapak, L. Conant, S.P. Goedegebuure, M.M. Berrien-Elliott et al., Memory-like differentiation enhances NK cell responses to Melanoma. Clin. Cancer Res. 27, 4859–4869 (2021)
L.D. Uppendahl, M. Felices, L. Bendzick, C. Ryan, B. Kodal, P. Hinderlie et al., Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against Ovarian cancer cells. Gynecol. Oncol. 153, 149–157 (2019)
J.N. Kochenderfer, S.A. Rosenberg, Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 10, 267–276 (2013)
C. Lin, J. Zhang, Reformation in chimeric antigen receptor based cancer immunotherapy: redirecting natural killer cell. Biochimica et Biophysica Acta (BBA) - reviews on Cancer. 2018;1869:200–215
M. Chmielewski, H. Abken, TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 15, 1145–1154 (2015)
L. Zhang, Y. Meng, X. Feng, Z. Han, CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark. Res. 10, 12 (2022)
Y. Gong, R.G.J. Klein Wolterink, J. Wang, G.M.J. Bos, W.T.V. Germeraad, Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 14, 73 (2021)
Y. Li, D.L. Hermanson, B.S. Moriarity, D.S. Kaufman, Human iPSC-Derived natural killer cells Engineered with chimeric Antigen receptors enhance anti-tumor activity. Cell. Stem Cell. 23, 181–192e5 (2018)
K.-Y. Teng, A.G. Mansour, Z. Zhu, Z. Li, L. Tian, S. Ma et al., Off-the-Shelf prostate Stem Cell Antigen–Directed chimeric Antigen receptor Natural Killer Cell Therapy to treat Pancreatic Cancer. Gastroenterology. 162, 1319–1333 (2022)
M. Gang, N.D. Marin, P. Wong, C.C. Neal, L. Marsala, M. Foster et al., CAR-modified memory-like NK cells exhibit potent responses to NK-resistant Lymphomas. Blood. 136, 2308–2318 (2020)
B. Cao, M. Liu, J. Huang, J. Zhou, J. Li, H. Lian et al., Development of mesothelin-specific CAR NK-92 cells for the treatment of gastric cancer. Int. J. Biol. Sci. 17, 3850–3861 (2021)
M. Liu, W. Huang, Y. Guo, Y. Zhou, C. Zhi, J. Chen et al., CAR NK-92 cells targeting DLL3 kill effectively small cell Lung cancer cells in vitro and in vivo. J. Leukoc. Biol. 2022
L. Wu, F. Liu, L. Yin, F. Wang, H. Shi, Q. Zhao et al., The establishment of polypeptide PSMA-targeted chimeric antigen receptor-engineered natural killer cells for castration-resistant Prostate cancer and the induction of ferroptosis-related cell death. Cancer Commun. (Lond) 2022
H. Liu, B. Yang, T. Sun, L. Lin, Y. Hu, M. Deng et al., Specific growth inhibition of ErbB2–expressing human Breast cancer cells by genetically modified NK–92 cells. Oncol. Rep. 33, 95–102 (2015)
R. Ma, T. Lu, Z. Li, K.-Y. Teng, A.G. Mansour, M. Yu et al., An oncolytic virus expressing IL-15/IL-15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 81, 3635–3648 (2021)
E. Liu, D. Marin, P. Banerjee, H.A. Macapinlac, P. Thompson, R. Basar et al., Use of CAR-Transduced Natural Killer cells in CD19-Positive lymphoid tumors. N Engl. J. Med. 382, 545–553 (2020)
CAR NK Cell Therapies Show Preliminary Safety, and Efficacy in AML, Non-Hodgkin Lymphoma [Internet]. OncLive. 2022 [cited 2023 Nov 9]. Available from: https://www.onclive.com/view/car-nk-cell-therapies-show-preliminary-safety-and-efficacy-in-aml-non-hodgkin-lymphoma
NKX019 Showcases Manageable Safety, Early Activity in R/R B-cell Non-Hodgkin Lymphoma [Internet]. OncLive. 2023 [cited 2023 Nov 9]. Available from: https://www.onclive.com/view/nkx019-showcases-manageable-safety-early-activity-in-r-r-b-cell-non-hodgkin-lymphoma
X. Zhang, Y. Guo, Y. Ji, Y. Gao, M. Zhang, Y. Liu et al., Cytokine release Syndrome after Modified CAR-NK Therapy in an Advanced Non-small Cell Lung Cancer patient: a Case Report. Cell Transpl. 31, 09636897221094244 (2022)
X. Wang, X. Yang, X. Yuan, W. Wang, Y. Wang, Chimeric antigen receptor-engineered NK cells: new weapons of cancer immunotherapy with great potential. Exp. Hematol. Oncol. 11, 85 (2022)
I. Nakase, N. Ueno, M. Matsuzawa, K. Noguchi, M. Hirano, M. Omura et al., Environmental pH stress influences cellular secretion and uptake of extracellular vesicles. FEBS Open. Bio. 11, 753–767 (2021)
A. Görgens, G. Corso, D.W. Hagey, R. Jawad Wiklander, M.O. Gustafsson, U. Felldin et al., Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles. 11, e12238 (2022)
L. Lugini, S. Cecchetti, V. Huber, F. Luciani, G. Macchia, F. Spadaro et al., Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 189, 2833–2842 (2012)
K. Kaban, C. Hinterleitner, Y. Zhou, E. Salva, A.G. Kantarci, H.R. Salih et al., Therapeutic silencing of BCL-2 using NK Cell-Derived exosomes as a Novel Therapeutic Approach in Breast Cancer. Cancers (Basel). 13, 2397 (2021)
Y. Qi, X. Zhao, Y. Dong, M. Wang, J. Wang, Z. Fan et al., Opportunities and challenges of natural killer cell-derived extracellular vesicles. Front. Bioeng. Biotechnol. 11, 1122585 (2023)
C.-H. Wu, J. Li, L. Li, J. Sun, M. Fabbri, A.S. Wayne et al., Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell. Vesicles. 8, 1588538 (2019)
F. Wu, M. Xie, M. Hun, Z. She, C. Li, S. Luo et al., Natural killer cell-derived extracellular vesicles: Novel players in Cancer Immunotherapy. Front. Immunol. 12, 658698 (2021)
A.M.L. Chan, J.M. Cheah, Y. Lokanathan, M.H. Ng, J.X. Law, Natural killer cell-derived extracellular vesicles as a Promising Immunotherapeutic Strategy for Cancer: a systematic review. Int. J. Mol. Sci. 24, 4026 (2023)
L. Zhu, S. Kalimuthu, J.M. Oh, P. Gangadaran, S.H. Baek, S.Y. Jeong et al., Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials. 190–191, 38–50 (2019)
M. Aarsund, F.M. Segers, Y. Wu, M. Inngjerdingen, Comparison of characteristics and Tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18. Cancer Immunol. Immunother 2022
P. Neviani, P.M. Wise, M. Murtadha, C.W. Liu, C.-H. Wu, A.Y. Jong et al., Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune Escape mechanisms. Cancer Res. 79, 1151–1164 (2019)
M. Zhang, W. Shao, T. Yang, H. Liu, S. Guo, D. Zhao et al., Conscription of Immune Cells by Light-Activatable Silencing NK-Derived Exosome (LASNEO) for Synergetic Tumor Eradication. Adv Sci (Weinh). 2022;e2201135
D. Han, K. Wang, T. Zhang, G.-C. Gao, H. Xu, Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur. Rev. Med. Pharmacol. Sci. 24, 5703–5713 (2020)
S. Rafiq, C.S. Hackett, R.J. Brentjens, Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020)
Y. Zhang, D.L. Wallace, de C.M. Lara, H. Ghattas, B. Asquith, A. Worth et al., In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral Infection. Immunology. 121, 258–265 (2007)
M. Daher, K. Rezvani, Outlook for New CAR-Based therapies with a focus on CAR NK cells: what lies Beyond CAR-Engineered T cells in the race against Cancer. Cancer Discov. 11, 45–58 (2021)
R.C. Sterner, R.M. Sterner, CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021)
Acknowledgements
Not applicable.
Funding
This work has been supported by the National Natural Science Foundation of China (82203163), the Natural Science Foundation of Hunan Province (2022JJ40660), the Natural Science Foundation of Changsha (kq2202123) and open sharing fund for the large-scale instruments and equipments of Central South University (CSUZC202235).
Author information
Authors and Affiliations
Contributions
MH, YXL, MP, JSG, YZM, YMW contributed to drafting and editing the manuscript and figures. WX, ZYZ and CMF designed, revised, and finalized the manuscript. YL and ZYZ participated in the design of this review. QJY and FYW contributed to the literature search. All authors contributed toward drafting and revising and agreed to submit.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, M., Liu, Y., Yan, Q. et al. NK cells as powerful therapeutic tool in cancer immunotherapy. Cell Oncol. 47, 733–757 (2024). https://doi.org/10.1007/s13402-023-00909-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00909-3