Abstract
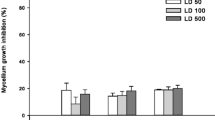
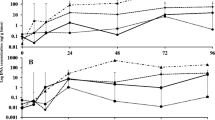
The present research was conducted to evaluate the marine algal extracts effectiveness on tomato Fusarium wilt disease. The organic extracts of macroalgae exhibited antagonistic activity against Fusarium oxysporum. The highest antifungal activity obtained from the methanolic extract of Cystoseira myrica followed by methanol extract of Sargassum cinereum. GC–mass analysis of some seaweed extracts was used to identify the presence of main compounds as dimethylocta-1,6-dien-3-ol,1-methoxy-4-(2-propenyl), hexadecanoic acid, methyl ester, and octadecanoic acid, and methyl ester. The infection of tomato plants with F. oxysporum induced a significant decrease in shoot and root dry weights as well as the photosynthetic pigments. There was a marked increase in soluble contents of saccharides and protein for infected tomato plant shoots and roots. On the other hand, pathogenicity stress induced a significant decrease in total contents of saccharides and protein of tomato shoots and roots. The results indicated a significant increase in total free amino acid content and antioxidant enzymes (CAT, POD, and APX) activities of inoculated tomato shoots and roots. The plant fresh and dry weights increased significantly by increasing its pigments content as a result of marine algal extracts application. On the other hand, algal extracts pretreatment decreased soluble saccharides and protein contents of plants, whereas increased significantly amino acid content in shoots of the inoculated plants. The increase in the activity of antioxidant enzymes played an essential role in increasing plant resistance against F. oxysporum. Finally, the marine macroalgae could serve as a new bioagent source for biological control of soil fungi.





Similar content being viewed by others
References
Recep, K.; Fikrettin, S.; Erkol, D.; Cafer, E.: Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol. Control 50, 194–198 (2009)
El-Sheekh, M.M.; Khairy, H.M.; El-Shenody, R.A.: Allelopathic effects of the cyanobacterium Microcystis aeruginosa on the growth and photosynthetic pigments of some algal species. Allelopathy J. 26(2), 275–290 (2010)
Skulberg, O.M.: Microalgal as a source of bioactive molecules—experience from cyanophyte research. Appl. Phycol. 12, 341–348 (2000)
Boobathy, S.; Soundarapandian, P.; Prithivraj, M.; Gunasundari, V.: Biochemical characterization of protein isolated from seaweed, Gracilaria edulis. Curr. Res. Biol. Sci. 2(1), 35–37 (2010)
Ismail, M.; El-Sheekh, M.: Enhancement of biochemical and nutritional contents of some cultivated seaweeds under laboratory conditions. J. Diet. Suppl. 15(3), 318–329 (2018)
El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.: Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 42(1), 65–74 (2016)
Gheda, S.; El-Sheekh, M.; Abou-Zeid, A.: In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pac. J. Trop. Med. 11(10), 583–589 (2018)
Rajasulochan, P.; Dhamotharan, R.; Krishnamoorthy, P.; Murugesan, S.: Antibacterial activity of the extracts of marine red and brown algae. Am. Sci. 5(3), 20–25 (2009)
Brimner, T.A.; Boland, G.J.: A review of the non-target effects of fungi used to biologically control plant diseases. Agric. Ecosyst. Environ. 100, 3–16 (2003)
Wang, Z.Y.; Li, D.B.: Study on Fusarium oxysporum pathogenesis vegetative compatible group. Zhejiang Agric. (in Chin.) 13(1), 72 (2001)
Morkuna, I.; Bednarski, W.; Kopyr, M.: Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 72, 167–178 (2008)
Dubey, S.C.; Suresh, M.; Singh, B.: Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol. Control 40, 118–127 (2007)
Dekker, J.: Acquired resistance to fungicides. Annu. Rev. Phytopathol. 14, 405–428 (1976)
Wongpia, A.; Lomthaisong, K.: Changes in the 2DE protein profiles of chilli pepper (Capsicum annuum) leaves in response to Fusarium oxysporum infection. Sci. Asia 36, 259–270 (2010)
Nagar, D.; Kumar, N.; Debbarama, R.; Reshma, V.S.: Investigation on the biochemical basis of resistance to Fusarium wilt in newly synthesized banana hybrids. Int. Q. J. Environ. Sci. IX, 593–596 (2016)
Venkatesh, K.V.; Kumar, K.G.; Pradeepa, K.; Kumar, S.R.; Kumar, R.S.: Biochemical markers assisted screening of Fusarium wilt resistant Musa paradisiaca (L.) cv. puttabale micropropagated clones. Ind. J. Exp. Biol. 51(7), 531–542 (2013)
Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B.: Sulfated macroalgal polysaccharides l-carrageenan and i-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 75, 38–45 (2010)
Patra, J.K.; Rath, S.K.; Jena, K.; Rathod, V.K.; Thatoi, H.: Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract, a study on inhibition of glutathione-S-Transferase activity. Turk. J. Biol. 32, 119–125 (2008)
El-Masry, H.A.; Fahmy, H.H.; Abdelwahed, A.S.H.: Synthesis and antimicrobial activity of some new benzimidazole derivatives. Molecules 5, 1429–1438 (2000)
Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J.: Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 166(2), 93–102 (2008)
Russell, A.D.; Hugo, W.B.; Ayliffe, G.A.J.: Principles and Practice of Disinfection, Preservation and Sterilization, p. 653. Blackwell, Boston (1982)
Metzner, H.; Rau, H.; Senger, H.: To synchronsiser bakeit investigations of individual pigment-deficiency mutants of Chlorella. Planta 65, 186–194 (1965)
Badour, S.S.A.: Analytically chemical investigation of the kaliummangles in Chlorella in comparison with other mangelezustanden. Ph.D. Dissertation, Goettingen, pp. 1–199 (1959)
Lowery, O.H.; Rasebrough, N.J.; Farr, A.L.; Randall, R.J.: Protein measurement with the Folin phenol reagent. Biol. Chem. 193, 291–297 (1951)
Moore, S.; Stein, W.W.: Amino acid free photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 176, 367–388 (1948)
Mukherjee, S.P.; Choudhuri, M.A.: Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Plant Physiol. 58, 166–170 (1983)
Aebi, H.: Catalase in vitro. Methods Enzymol. 105, 121–126 (1984)
Havir, E.A.; Mellate, N.A.: Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 84, 450–455 (1987)
Klapheck, S.; Zimmer, I.; Cosse, H.: Scavenging of hydrogen peroxide in endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol. 31, 1005–1013 (1990)
Asada, K.; Chen, G.: On activation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant Cell Physiol. 33, 117–123 (1992)
Kolanjinathan, K.; Stella, D.: Antibacterial activity of marine macro algae against human pathogens. Recent Res. Sci. Technol. 1(1), 20–22 (2009)
Omar, H.H.; Gumgumji, N.M.; Shiek, H.M.; El-Kazan, M.M.; El-Gendy, A.M.: Inhibition of the development of pathogenic fungi by extracts of some marine algae from the Red Sea of Jeddah, Saudi Arabia. Afr. J. Biotechnol. 11(72), 13697–13704 (2012)
Soliman, ASh; Ahmed, A.Y.; Abdel-Ghafour, S.E.; El-Sheekh, M.M.; Sobhy, H.M.: Antifungal bio-efficacy of the red algae Gracilaria confervoides extracts against three pathogenic fungi of cucumber plant. Middle East J. Appl. Sci. 8(3), 727–735 (2018)
Dikmen, M.; Ozturk, N.; Ozturk, Y.: The antioxidant potency of Punica granatum L. fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J. Med. Foods 14, 1638–1646 (2011)
Ceballos, R.; Cofré, X.; Quiroz, A.; Espinoza, N.; Cofré, X.: Bentazon-MCPA effect on Fusarium oxysporum root rot on Trifolium pratense in greenhouse conditions. J. Soil Sci. Plant Nutr. 9(2), 142–154 (2009)
El-Khallal, S.M.: Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (Arbuscular Mycorrhiza) and/or hormonal elicitors (jasmonic acid and salicylic acid), 1—changes in growth, some metabolic activities and endogenous hormones related to defense mechanism. Aust. Basic Appl. Sci. 1(4), 691–705 (2007)
Nafie, E.M.: The possible induction of resistance in Lupinus termis L. against Fusarium oxysporum by Streptomyces chibaensis and its mode of action, I. Changes in certain morphological criteria and biochemical composition related to induced resistance. Int. Agric. Biol. 5(4), 463–472 (2003)
Nemec, S.: Stress-related compounds in xylem fluid of blight-diseased citrus containing Fusarium solani nophthazarin toxins and their effect on the host. Can. J. Microbiol. 41, 515–524 (1995)
Jayaraj, J.; Wan, A.; Rahman, M.; Punja, Z.K.: Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 27, 1360–1366 (2008)
Pise, N.M.; Sabale, A.B.: Effect of seaweed concentrates on the growth and biochemical constituents of Trigonella foenum-graecum L. J. Phytol. 2(4), 50–56 (2010)
Hashem, M.; Hamada, A.M.: Evaluation of two biologically active compounds for control of wheat root rot and its causal pathogens. Mycobiology 30(4), 233–239 (2002)
Al-Hakimi, A.M.A.; Alghalibi, S.M.S.: Thiamin and salicylic acid as biological alternatives for controlling broad been rot. Appl. Sci. Environ. Manag. 11(4), 125–131 (2007)
Thirumaran, G.; Arumugam, M.; Arumugam, R.; Anantharaman, P.: Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (I) Medikus. Am. Euras. J. Agron. 2, 57–66 (2009)
Heiser, I.; Obwald, W.; Elstner, E.: The formation of reaction oxygen species by fungal and bacterial phytoxins. Plant Physiol. Biochem. 36, 703–713 (1998)
Bishop, D.L.; Chatterton, N.J.; Harrison, P.A.; Hatfield, R.D.: Changes in carbohydrate coordinated partitioning and cell wall remodeling with stress-induced pathogenesis in wheat sheaths. Physiol. Mol. Plant 61, 53–63 (2002)
Vafaii, A.A.; Ketabchi, S.; Moradshahi, A.: Effect of benzo (1,2,3) thiadiazole-7-carbothioic acid and S-methyl ester (BTH) on biochemical responses of wheat seedlings infected by Fusarium culmorum. Int. J. Farm. Allied Sci. 2(12), 334–342 (2013)
Arad, S.M.; Richmond, A.E.: Leaf cell water and enzyme activity. Plant Physiol. 57(4), 656–658 (1976)
Abu-Taleb, A.M.; Al-Mousa, A.A.: Evaluation of the antifungal activity of vitavax and Trichoderma viride against two wheat root rot pathogens. Appl. Biosci. 6, 140–149 (2008)
Matthäus, K.; Dunicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.: Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 58(1), 19–35 (2004)
Kharazian, Z.A.; Aghdasi, M.; Jouzan, G.S.; Zamani, M.: Effects of Fusarium verticillioides and lactobacillus strains inoculation on growth and antioxidant enzymes activity of Zea mays plants. J. Hortic. Res. 25(2), 67–74 (2017)
Anand, T.; Bhaskaran, R.; Raguchander, T.; Samiyappan, R.; Prakasam, V.; Gopalakrishnan, C.: Defence responses of chilli fruits to Colletotrichum capsici and Alternaria alternate. Biol. Plant. 53(3), 553–559 (2009)
Dignum, M.J.W.; Kerler, J.; Verpoorte, R.: B-Glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochem. Anal. 12, 174–179 (2001)
Hammerschmidt, R.; Nuckles, E.; Kuc, J.: Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Phys. Plant Physiol. 20, 73–80 (1982)
Cawood, M.E.; Pretorius, J.C.; Westhuizen, A.J.V.; Pretorius, Z.A.: Disease development and PR-protein activity in wheat (Triticum aestivum) seedlings treated with plant extracts prior to leaf rust (Puccinia triticina) infection. Crop Prot. 29(11), 1311–1319 (2010)
Liu, X.; Huang, B.: Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 40, 503–510 (2000)
Anjum, T.; Fatima, S.; Amjad, S.: Physiological changes in wheat during development of loose smut. Trop. Plant Pathol. 37(2), 102–107 (2012)
Lizzi, Y.; Coulomb, C.; Polian, C.; Coulomb, P.J.; Coulomb, P.O.: Seaweed and mildew, what does the future hold? Encouraging laboratory results. Phytoma Def. Plants 508, 29–30 (1998)
Cox, S.; Abu-Ghannam, N.; Gupta, S.: An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 17, 205–220 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Sheekh, M.M., Mousa, A.S.H. & Farghl, A.A.M. Biological Control of Fusarium Wilt Disease of Tomato Plants Using Seaweed Extracts. Arab J Sci Eng 45, 4557–4570 (2020). https://doi.org/10.1007/s13369-020-04518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04518-2