Abstract
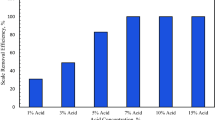
Barite scale is the most common oilfield scale in oil and gas wells. Barite scale removal still represents a challenge to the oil and gas industry because of its low solubility in mineral acids such as HCl. Chelating agents such as diethylene triamine pent acetic acid (DTPA), ethylene diamine tetra acetic acid (EDTA), and hydroxy ethyl ethylene tri acetic acid were introduced as barite scale removers, but the solubility of barite in these chelates was very low. Huge amount of chelating agents are required to remove the barite scale, but we are constrained by the hole volume of the well. In this study, a novel formulation was developed to dissolve the barite scale by using chelating agents and a catalyst to enhance the solubility of barite in chelating agent (DTPA) with small volume of chelating agents. Using the catalyst in this formulation is the main feature of the treatment. Solubility experiments were conducted to determine the dissolution rate of barite in DTPA chelating agent at different temperatures. The effect of pH on the dissolution rate of barite was studied using two bases (potassium base KOH and sodium base NaOH). The optimum chelating agent (DTPA) concentration and time were identified that will achieve the maximum barite scale solubility. Also, the catalyst type and concentration were determined using high-temperature high-pressure solubility experiments. Kinetics experiments using rotating disk apparatus were performed to investigate the mechanism of catalyst during the reaction of DTPA with barite. Based on the results from this study, DTPA (0.5M concentration) potassium base chelating agent was able to dissolve 60% of barite scale at high pH (11–12) in 24 h. The solubility of barite in the DTPA increased with time up to 24 h and after that it remained constant which means the optimum time for the barite scale removal is 24 h. The removal of barite scale increased up to 80–90% after adding (5–7 wt %) of one of the following catalyst: potassium carbonate \((\hbox {K}_{2}\hbox {CO}_{3})\), potassium chloride (KCl), and potassium formate \((\hbox {CHKO}_{2})\). The developed formulation (DTPA/catalyst) can be used to remove the barite scale from oil and gas wells effectively. Rotating disk experiments showed that the catalyst enhanced the DTPA diffusion to the rock surface and in turn enhanced the mass transfer reaction rate.
Similar content being viewed by others
Abbreviations
- \(W_{\mathrm{sc}}\) :
-
Weight of the scale
- \(T_{d}\) :
-
Inside diameter of tubing before the scale formed
- \(F_{d}\) :
-
Inside diameter of tubing after the scale formed
- \(\rho _{\mathrm{sc}}\) :
-
Density of the scale
- \(V_{\mathrm{f}}\) :
-
Fluid volume in the tubing after the scale formed
- \(h_{\mathrm{f}}\) :
-
Heterogeneity factor
- k :
-
Core permeability, md
- \(k_{\mathrm{c}}\) :
-
Mass transfer coefficient (cm/s)
- DTPA:
-
Diethylenetriaminepentaacetic acid
- KOH:
-
Potassium hydroxide
- NaOH:
-
Sodium hydroxide
- \(\mathrm{K}_{2}\mathrm{CO}_{3}\) :
-
Potassium carbonate
- KCl:
-
Potassium chloride
- \(\hbox {CHKO}_{2}\) :
-
Potassium formate
- g/l:
-
Gram per liter
References
Dreher, T.; Biley, A.; Tuckett, P.: Stop erosion at the well head on fractured wells. Paper OTC-24724-MS Presented at the Offshore Technology Conference-Asia, Kuala Lumpur, Malaysia, 25–28 March (2014)
Clemmits, A.F.; Ballance, D.C.; Hunton, A.G.: The dissolution of scales in oilfield systems. Paper SPE 14010 Presented at the SPE Offshore Conference held in Aberdeen, Scotland, 10–13 September (1985)
Morris, R.L.; Paul, J.M.: Method for removing sulfate scale. US Patent 4,980,077 (1990)
Wang, K.S.; et al.: Dissolution of the Barite (001) surface by the chelating agent DTPA as studied with non- contact atomic force microscopy. J. Colloids Surf. A Physicochem. Eng. Asp. 160, 217–227 (1999)
Nasr-El-Din, H. A.; Al-Mutairi, S.H.; Al-Hajji, H.H.: Evaluation of a new barite dissolver: lab studies. SPE 86501, Presented at the SPE International Symposium and Exhibition on Formation Damage Control Held in Lafayette, Louisiana, USA, 18–20 February (2004)
Mahmoud, M.A.; Nasr-El-Din, H.A.: Modeling flow of chelating agents during stimulation of carbonate reservoirs. Arab. J. Sci. Eng. 39, 9239–9248 (2014). doi:10.1007/s13369-014-1437-4
Bageri, B.; Mahmoud, M.A.: A new diversion technique to remove the formation damage from maximum reservoir contact and extended reach wells in sandstone reservoirs. Paper SPE 165163 Presented at the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands 5–7 June (2013)
Mahmoud, M.A.; Abdelgawad, K.Z.: A new chemical EOR for sandstone and carbonate reservoirs. Paper SPE 172183 Presented at the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 21–24 April (2014)
Abdelgawad, K.Z.; Mahmoud, M.A.: High-performance EOR system in carbonate reservoirs. Paper SPE 172182 Presented at the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 21–24 April (2014)
Al-Zahrani, A.R.; et al.: Utilizing chelating agent system fluid to remove scale buildup from stuck ESP shaft in offshore Saudi Arabia. Paper SPE 168093 Presented at the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 19–22 May (2014)
Mahmoud, M.A.: Evaluating the damage caused by calcium sulfate scale precipitation during low- and high-salinity-water injection. J. Can. Petrol. Technol. 53(3), 141–150 (2014)
Lakatos, I.; et al.: Optimization of barite dissolvers by organic acids and pH regulation. Paper SPE 74667 Presented at the SPE Oilfield Scale Symposium Held in Aberdeen, Scotland, 30–31 February (2002)
Lakatos, I.; Lakatos-Szabo, J.; Kosztin, B.: Comparative study of different barite dissolvers: technical and economic aspects. Paper SPE 73719 Presented at International Symposium and Exhibition on Formation Damage Control, Lafayette, Louisiana, 20–21 February (2002)
Putnis, C.V.; Kowacz, M.; Putnis, A.: The mechanism and kinetics of DTPA-promoted dissolution of barite. J. Appl. Geochem. 23, 2778–2788 (2008)
Putnis, A.; Putnis, C.V.; Paul, J.M.: The efficiency of a DTPA-based solvent in the dissolution of barium sulfate scale deposits. Paper SPE 29094 Presented at the International Symposium on Oilfield Chemistry, San Antonio, Texas, 14–17 February (1995)
Paul, J.M.; Fieler, E.R.: A new solvent for oilfield scale. Paper SPE 24847 Presented at the SPE Annual Technical Conference and Exhibition held in Washington, DC, 4–7 October (1992)
Frenier, W.W.: Novel scale removers are developed for dissolving alkaline earth deposits. Paper SPE 65027 Presented at the 2001 SPE Oilfield Scale held in Aberdeen, Scotland, 30–31 January (2001)
Putnis, C.V.; Kowacz, M.; Putnis, A.: The mechanism and kinetics of DTPA-promoted dissolution of barite. J. Appl. Geochem. 23, 2778–2788 (2008)
Jordan, M. M.; Williams, H.; Linares-Samaniego, S.; Frigo, D.M.: New insights on the impact of high temperature conditions (\(176^{\circ }\text{C}\)) on carbonate and sulphate scale dissolver performance. Paper SPE 169785-MS Presented at the SPE International Oilfield Scale Conference and Exhibition, Aberdeen, Scotland, 14–15 May (2014)
Levich, V.G.: Physicochemical Hydrodynamics (Transl. by Scripta Technica, inc.). Prentice-Hall, Upper Saddle River (1962)
Newman, J.: Schmidt number correction for the rotating disk. J. Phys. Chem. 70(4), 1327–1328 (1966)
Hunton, A.G.: Improvements in scale dissolver formulations. In: Proceeding, \(5^{{\rm th}}\) Oilfield Chemistry Symposium, Geilo, Norway (1994)
Dunn, K.; Yen, T.F.; Shuler, P.J.; Tang, Y.: Chemical dissolution for controlling barium sulfate scale deposit. Am. Chem. Soc. Div. Pet. Chem. 42(3), 691–693 (1997)
Keatch, R.: Method for dissolving oilfield scale. Patent, US 2007/0221246 A1 (2007)
Dunn, K.; Yen, T.F.: Dissolution of barium sulfate scale deposits by chelating agents. J. Environ. Sci. Technol 33(16), 2821–2824 (1999). doi:10.1021/es980968j
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bageri, B.S., Mahmoud, M.A., Shawabkeh, R.A. et al. Toward a Complete Removal of Barite (Barium Sulfate \(\hbox {BaSO}_{4}\)) Scale Using Chelating Agents and Catalysts. Arab J Sci Eng 42, 1667–1674 (2017). https://doi.org/10.1007/s13369-017-2417-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-017-2417-2