Abstract
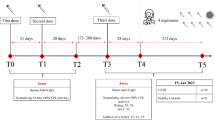
The effectiveness of COVID-19 vaccination is still unclear in individuals with underlying diseases such as HTLV-1 infection. This retrospective cohort study aimed to evaluate the humoral response of COVID-19 vaccines among people living with HTLV-1 (PLHTLV) in northeastern Iran. From December 2021 to October 2022, eighty-six HTLV-1+ subjects (50 males and 36 females; 47.7 ± 11.2 years) and 90 HTLV-1 seronegative individuals (age- and sex-matched convenient samples) were enrolled. The humoral immune response was evaluated by measuring different COVID-19 Abs in serum samples at least 28 days after receiving 2nd or 3rd doses of COVID-19 vaccines. Throughout all three rounds of immunization, Sinopharm was the most commonly used COVID-19 vaccine across all three immunization rounds. Compared to the HTLV-1− group, a significantly lower frequency of all four Abs activity was observed among PLHTLV:anti-nucleocapsid (66.3% vs 86.7%, p = 0·001), anti-spike (91.9% vs 98.9%, p = 0·027), RBD (90.7% vs 97.8%, p = 0·043), and neutralizing Abs (75.6% vs 95.5%, p < 0·001). Also, the frequency of all Abs in 28 patients with HAM/TSP was higher than that of 58 asymptomatic carriers, although this difference was statistically significant only in the case of anti-spike Abs (p = 0.002). Notably, PLHTLV-vaccinated against COVID-19 demonstrated significantly lower antibody activities, indicating a reduced humoral immune response to COVID-19 vaccines.
Similar content being viewed by others
Availability of data and materials
All data supporting this study's findings is included in the manuscript and available from the corresponding author upon request.
References
Araujo A, Martin F (2020) Human T leukaemia Type 1 and COVID-19. Pathogens 9:438
Araujo A, Martin F (2022) SARS-CoV-2 vaccination of people living with human T leukaemia virus type 1. Sex Transm Infect 98:154
Dashdorj NJ, Wirz OF, Roltgen K, Haraguchi E, Buzzanco AS 3rd, Sibai M, Wang H, Miller JA, Solis D, Sahoo MK, Arunachalam PS, Lee AS, Shah MM, Liu J, Byambabaatar S, Bat-Ulzii P, Enkhbat A, Batbold E, Zulkhuu D, Ochirsum B, Khurelsukh T, Dalantai G, Burged N, Baatarsuren U, Ariungerel N, Oidovsambuu O, Bungert AS, Genden Z, Yagaanbuyant D, Mordorj A, Pulendran B, Chinthrajah S, Nadeau KC, Jardetzky T, Wilbur JL, Wohlstadter JN, Sigal GB, Pinsky BA, Boyd SD, Dashdorj ND (2021) Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe 29:1738–1743
Fu Y, Chen F, Cui L, Zhao Y, Zhang H, Fu S, Zhang J (2021) Immunological analysis of people in Northeast China after SARS-CoV-2 inactivated vaccine injection. Vaccines 9:1028
Futsch N, Mahieux R, Dutartre H (2017) HTLV-1, the other pathogenic yet neglected human retrovirus: from transmission to therapeutic treatment. Viruses 10:1
Gonçalves DU, Proietti FA, Ribas JGR, Araújo MG, Pinheiro SR, Guedes AC, Carneiro-Proietti ABF (2010) Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev 23:577–589
Gouya MM, Seif-Farahi K, Hemmati P (2023) An overview of Iran’s actions in response to the COVID-19 pandemic and in building health system resilience. Front Public Health 11:1073259
Heidari M, Sayfouri N, Jafari H (2022) Consecutive Waves of COVID-19 in Iran: Various Dimensions and Probable Causes. Disaster Med Public Health Prep 17:e136
Herzog Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L, Benveniste-Levkovitz P, Bar Chaim A, Koren-Michowitz M (2021) BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol 96:1195–1203
Hirons A, Khoury G, Purcell DF (2021) Human T-cell lymphotropic virus type-1: A lifelong persistent infection, yet never truly silent. Lancet Infect Dis 21:e2–e10
Holt SG, Mahmoud S, Ahmed W, Acuna JM, Al Madani AK, Eltantawy I, Zaher WA, Goodier GJ, Al Kaabi NA, Al Obaidli AA (2022) An analysis of antibody responses and clinical sequalae of the Sinopharm HB02 COVID19 vaccine in dialysis patients in the United Arab Emirates. Nephrology (carlton) 27:260–268
Kak G, Raza M, Tiwari BK (2018) Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol Concepts 9:64–79
Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, Khan ST (2020) COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules 26:39
Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, Tay SH, Teo CB, Tan BKJ, Chan YH, Sundar R, Soon YY (2022) Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 376:e068632
Lijeskić O, Klun I, Stamenov Djaković M, Gligorić N, Štajner T, Srbljanović J, Djurković-Djaković O (2021) Prospective cohort study of the kinetics of specific antibodies to SARS-CoV-2 infection and to four SARS-CoV-2 vaccines available in Serbia, and vaccine effectiveness: a 3-month interim report. Vaccines 9:1031
Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE (2021) Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. The Lancet HIV 8:e568–e580
Moss P (2022) The T cell immune response against SARS-CoV-2. Nat Immunol 23:186–193
Paludan SR, Mogensen TH (2022) Innate immunological pathways in COVID-19 pathogenesis. Sci Immunol 7:eabm5505
Quaresma JA, Yoshikawa GT, Koyama RV, Dias GA, Fujihara S, Fuzii HT (2015) HTLV-1, immune response and autoimmunity. Viruses 8:5
Sajjadi S, Hejazi S, Ravanshad S, Jafarzadeh Esfehani R (2022) Human T-lymphotropic virus type 1 and novel coronavirus disease 2019; More complex than just a simple coinfection. Gene 834:146550
Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J (2020) Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 20:133–143
Ssentongo P, Heilbrunn ES, Ssentongo AE, Advani S, Chinchilli VM, Nunez JJ, Du P (2021) Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep 11:6283
Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, Gonzalez CJ, Udo T, Morne JE, Hart-Malloy R (2021) COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open 4:e2037069
Tian L, Elsheikh EB, Patrone PN, Kearsley AJ, Gaigalas AK, Inwood S, Lin-Gibson S, Esposito D, Wang L (2021) Towards quantitative and standardized serological and neutralization assays for COVID-19. Int J Mol Sci 22:2723
Willems L, Hasegawa H, Accolla R, Bangham C, Bazarbachi A, Bertazzoni U, de Freitas Carneiro-Proietti AB, Cheng H, Chieco-Bianchi L, Ciminale V (2017) Reducing the global burden of HTLV-1 infection: an agenda for research and action. Antiviral Res 137:41–48
World Health Organization (2023) Available from: https://covid19.who.int/region/emro/country/ir. Accessed 27 Aug 2023
Yau K, Abe KT, Naimark D, Oliver MJ, Perl J, Leis JA, Bolotin S, Tran V, Mullin SI, Shadowitz E (2021) Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw Open 4:e2123622–e2123622
Acknowledgements
The authors especially thank the Vice-Chancellor of Research and Technology, Iranian Academic Center for Education, Culture, and Research (ACECR) for granted this study. The authors thank the Vice-Chancellor of Medicine, ACECR-Razavi Khorasan, Mashhad, Iran for their valuable help and support.
Funding
This study was supported financially by the Vice-Chancellor of Research and Technology, Iranian Academic Center for Education, Culture, and Research (ACECR), under Grant [ACECR 3101–20].
Author information
Authors and Affiliations
Contributions
Conception and design of the study: RJE, AM, HR, and HRB; Performing the experiments: ZV, MS, AM, AS and MS; Research director and data analysis: MRH; Research advisors: HR and RB; Drafting, revision and finalization of the manuscript: RJE and MRH. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jafarzadeh Esfehani, R., Vahidi, Z., Shariati, M. et al. Immune response to COVID-19 vaccines among people living with human T-cell lymphotropic virus type 1 infection: a retrospective cohort study from Iran. J. Neurovirol. (2023). https://doi.org/10.1007/s13365-023-01176-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13365-023-01176-6