Abstract
Central nervous system (CNS) tumors are the most frequent solid malignant tumors in pediatric patients and are the leading cause of tumor-related death in children. Treatment for this heterogeneous group of tumors consists of various combinations of safe maximal surgical resection, chemotherapy, and radiation therapy which offer a cure for some children but often cause debilitating adverse late effects in others. While therapies targeting the tumor microenvironment (TME) like immune checkpoint inhibition (ICI) have been successful in treating some cancers, these therapies failed to exhibit treatment efficacy in the majority of pediatric brain tumors in the clinic. Importantly, the pediatric TME is unique and distinct from adult brain tumors and designing therapies to effectively target these tumors requires understanding the unique biology of pediatric brain tumors and the use of translational models that recapitulate the TME. Here we describe the TME of medulloblastoma (MB) and diffuse midline glioma (DMG), specifically diffuse intrinsic pontine glioma (DIPG), and further present the current drug delivery approaches and clinical administration routes targeting the TME in these tumors, including preclinical and clinical studies.
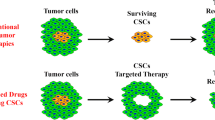
Graphical Abstract
Considerations for developing therapeutic approaches to target pediatric brain tumor microenvironment including drug delivery carriers, CNS specific delivery routes, and clinically relevant models for preclinical evaluation 1.




Similar content being viewed by others
Data availability
Not applicable.
References
Graphical Abstract: Created in BioRender. Stockwell, C. 2025. https://BioRender.com/l62r123.
Ligon JA, Sayour EJ. Spotlighting cellular therapies to advance the treatment of medulloblastoma. Neuro Oncol. 2023;25:628–30. https://doi.org/10.1093/neuonc/noad005.
Baroni LV, Sampor C, Gonzalez A, et al. Bridging the treatment gap in infant medulloblastoma: molecularly informed outcomes of a globally feasible regimen. Neuro Oncol. 2020;22:1873–81. https://doi.org/10.1093/neuonc/noaa122.
Shaik S, Maegawa S, Gopalakrishnan V. Medulloblastoma: novel insights into emerging therapeutic targets. Expert Opin Ther Targets. 2021;25:615–9. https://doi.org/10.1080/14728222.2021.1982896.
van Bree NFHN, Wilhelm M. The tumor microenvironment of medulloblastoma: an intricate multicellular network with therapeutic potential. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14205009.
Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130:815–27. https://doi.org/10.1007/s00401-015-1478-0.
Gállego Pérez-Larraya J, Garcia-Moure M, Labiano S, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N Engl J Med. 2022;386:2471–81. https://doi.org/10.1056/NEJMoa2202028.
Ehteda A, Khan A, Rajakumar G, et al. Microtubule-targeting combined with HDAC inhibition is a novel therapeutic strategy for diffuse intrinsic pontine gliomas. Mol Cancer Ther. 2023. https://doi.org/10.1158/1535-7163.MCT-23-0179.
Chen Y, Zhao C, Li S, et al. Immune microenvironment and immunotherapies for diffuse intrinsic pontine glioma. Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15030602.
O’Keeffe E, Campbell M. Modulating the paracellular pathway at the blood-brain barrier: current and future approaches for drug delivery to the CNS. Drug Discov Today Technol. 2016;20:35–9. https://doi.org/10.1016/j.ddtec.2016.07.008.
de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. https://doi.org/10.1016/j.ccell.2023.02.016.
Ozkaya T, Chien F, Boya M, et al. Biom-62. circulating tumor cells in medulloblastoma: a novel peripheral biomarker for cns disease. Neuro Oncol. 2024;26:viii34–viii34. https://doi.org/10.1093/neuonc/noae165.0134.
Stockwell, C. Figure 1: created in BioRender. 2025. https://www.BioRender.com/j13k353. Accessed 12 Mar 2025.
Ballester LY, Wang Z, Shandilya S, et al. Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol. 2013;37:1357–64. https://doi.org/10.1097/PAS.0b013e318294e817.
Liu G, Qiu Y, Zhang P, et al. Immunogenic cell death enhances immunotherapy of diffuse intrinsic pontine glioma: from preclinical to clinical studies. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14091762.
Arrillaga-Romany I, Lassman A, McGovern SL, et al. ACTION: a randomized phase 3 study of ONC201 (dordaviprone) in patients with newly diagnosed H3 K27M-mutant diffuse glioma. Neuro Oncol. 2024;26:S173–81. https://doi.org/10.1093/neuonc/noae031.
Venneti S, Kawakibi AR, Ji S, et al. Clinical Efficacy of ONC201 in H3K27M-mutant diffuse midline gliomas is driven by disruption of integrated metabolic and epigenetic pathways. Cancer Discov. 2023;13:2370–93. https://doi.org/10.1158/2159-8290.CD-23-0131.
Bailey CP, Wang R, Figueroa M, et al. Computational immune infiltration analysis of pediatric high-grade gliomas (pHGGs) reveals differences in immunosuppression and prognosis by tumor location. Comp Sys Onco. 2021;1. https://doi.org/10.1002/cso2.1016.
Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. https://doi.org/10.1007/s00401-016-1545-1.
Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51. https://doi.org/10.1093/neuonc/noab106.
Larson JD, Kasper LH, Paugh BS, et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell. 2019;35:140-155.e7. https://doi.org/10.1016/j.ccell.2018.11.015.
Jain SU, Do TJ, Lund PJ, et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019;10:2146. https://doi.org/10.1038/s41467-019-09981-6.
Lewis PW, Müller MM, Koletsky MS, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–61. https://doi.org/10.1126/science.1232245.
Hervás-Corpión I, Gallardo-Orihuela A, Catalina-Fernández I, et al. Potential diagnostic value of the differential expression of histone H3 variants between low- and high-grade gliomas. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13215261.
Zhu X, Lazow MA, Schafer A, et al. A pilot radiogenomic study of DIPG reveals distinct subgroups with unique clinical trajectories and therapeutic targets. Acta Neuropathol Commun. 2021;9:14. https://doi.org/10.1186/s40478-020-01107-0.
Ross JL, Puigdelloses-Vallcorba M, Piñero G, et al. Microglia and monocyte-derived macrophages drive progression of pediatric high-grade gliomas and are transcriptionally shaped by histone mutations. Immunity. 2024;57:2669-2687.e6. https://doi.org/10.1016/j.immuni.2024.09.007.
Andrade AF, Annett A, Karimi E, et al. Immune landscape of oncohistone-mutant gliomas reveals diverse myeloid populations and tumor-promoting function. Nat Commun. 2024;15:7769. https://doi.org/10.1038/s41467-024-52096-w.
Lin GL, Nagaraja S, Filbin MG, et al. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2018;6:51. https://doi.org/10.1186/s40478-018-0553-x.
Ross JL, Velazquez Vega J, Plant A, et al. Tumour immune landscape of paediatric high-grade gliomas. Brain. 2021;144:2594–609. https://doi.org/10.1093/brain/awab155.
Liu I, Jiang L, Samuelsson ER, et al. The landscape of tumor cell states and spatial organization in H3–K27M mutant diffuse midline glioma across age and location. Nat Genet. 2022;54:1881–94. https://doi.org/10.1038/s41588-022-01236-3.
Robinson MH, Vasquez J, Kaushal A, et al. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J Immunother Cancer. 2020;8. https://doi.org/10.1136/jitc-2020-001066.
Plant AS, Koyama S, Sinai C, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol. 2018;137:269–78. https://doi.org/10.1007/s11060-017-2737-9.
Lee C, Lee J, Choi SA, et al. M1 macrophage recruitment correlates with worse outcome in SHH medulloblastomas. BMC Cancer. 2018;18:535. https://doi.org/10.1186/s12885-018-4457-8.
Lieberman NAP, DeGolier K, Kovar HM, et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 2019;21:83–94. https://doi.org/10.1093/neuonc/noy145.
Bockmayr M, Klauschen F, Maire CL, et al. Immunologic profiling of mutational and transcriptional subgroups in pediatric and adult high-grade gliomas. Cancer Immunol Res. 2019;7:1401–11. https://doi.org/10.1158/2326-6066.CIR-18-0939.
Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573:532–8. https://doi.org/10.1038/s41586-019-1564-x.
Venkatesh HS, Johung TB, Caretti V, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:803–16. https://doi.org/10.1016/j.cell.2015.04.012.
Krishna S, Choudhury A, Keough MB, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. 2023;617:599–607. https://doi.org/10.1038/s41586-023-06036-1.
Winkler F, Venkatesh HS, Amit M, et al. Cancer neuroscience: state of the field, emerging directions. Cell. 2023;186:1689–707. https://doi.org/10.1016/j.cell.2023.02.002.
Drexler R, Khatri R, Sauvigny T, et al. A prognostic neural epigenetic signature in high-grade glioma. Nat Med. 2024;30:1622–35. https://doi.org/10.1038/s41591-024-02969-w.
Goethe EA, Deneen B, Noebels J, Rao G. The role of hyperexcitability in gliomagenesis. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms24010749.
Barron T, Yalcin B, Mochizuki A, et al. GABAergic neuron-to-glioma synapses in diffuse midline gliomas. BioRxiv. 2022. https://doi.org/10.1101/2022.11.08.515720.
Barron T, Yalçın B, Mochizuki A, et al. Cnsc-01. gabaergic neuron-to-glioma synapses in diffuse midline gliomas. Neuro Oncol. 2023;25:i11–i11. https://doi.org/10.1093/neuonc/noad073.044.
Venkatesh HS, Tam LT, Woo PJ, et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. 2017;549:533–7. https://doi.org/10.1038/nature24014.
Taylor KR, Barron T, Hui A, et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature. 2023;623:366–74. https://doi.org/10.1038/s41586-023-06678-1.
Trombetta-Lima M, Rosa-Fernandes L, Angeli CB, et al. Extracellular matrix proteome remodeling in human glioblastoma and medulloblastoma. J Proteome Res. 2021;20:4693–707. https://doi.org/10.1021/acs.jproteome.1c00251.
Loveson K, Fillmore H. Dipg-57. a comprehensive gene/protein investigation of the tumour microenvironment in diffuse midline glioma in children. Neuro Oncol. 2018;20:i60–i60. https://doi.org/10.1093/neuonc/noy059.150.
Qi J, Esfahani DR, Huang T, et al. Tenascin-C expression contributes to pediatric brainstem glioma tumor phenotype and represents a novel biomarker of disease. Acta Neuropathol Commun. 2019;7:75. https://doi.org/10.1186/s40478-019-0727-1.
Fu Z, Zhu G, Luo C, et al. Matricellular protein tenascin C: implications in glioma progression, gliomagenesis, and treatment. Front Oncol. 2022;12:971462. https://doi.org/10.3389/fonc.2022.971462.
Qi J, Huang T, Hashizume R, et al. Dipg-25. in vitro and in vivo analysis of tenascin-c expression in pediatric brainstem glioma. Neuro Oncol. 2017;19:iv10–iv10. https://doi.org/10.1093/neuonc/nox083.040.
Zhang J, Wang T. Immune cell landscape and immunotherapy of medulloblastoma. Pediatr Investig. 2021;5:299–309. https://doi.org/10.1002/ped4.12261.
Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14:1200–7. https://doi.org/10.1016/S1470-2045(13)70449-2.
Van Ommeren R, Garzia L, Holgado BL, et al. The molecular biology of medulloblastoma metastasis. Brain Pathol. 2020;30:691–702. https://doi.org/10.1111/bpa.12811.
Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral Heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:737-754.e6. https://doi.org/10.1016/j.ccell.2017.05.005.
Luzzi S, Giotta Lucifero A, Brambilla I, et al. Targeting the medulloblastoma: a molecular-based approach. Acta Biomed. 2020;91:79–100. https://doi.org/10.23750/abm.v91i7-S.9958.
Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–9. https://doi.org/10.1038/nature09587.
Hovestadt V, Smith KS, Bihannic L, et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature. 2019;572:74–9. https://doi.org/10.1038/s41586-019-1434-6.
Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. https://doi.org/10.1007/s00401-011-0922-z.
Williamson D, Schwalbe EC, Hicks D, et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022;40:111162. https://doi.org/10.1016/j.celrep.2022.111162.
Riemondy KA, Venkataraman S, Willard N, et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro Oncol. 2022;24:273–86. https://doi.org/10.1093/neuonc/noab135.
Vladoiu MC, El-Hamamy I, Donovan LK, et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature. 2019;572:67–73. https://doi.org/10.1038/s41586-019-1158-7.
Göbel C, Godbole S, Schoof M, et al. MYC overexpression and SMARCA4 loss cooperate to drive medulloblastoma formation in mice. Acta Neuropathol Commun. 2023;11:174. https://doi.org/10.1186/s40478-023-01654-2.
Ramaswamy V, Remke M, Adamski J, et al. Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro Oncol. 2016;18:291–7. https://doi.org/10.1093/neuonc/nou357.
Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–84. https://doi.org/10.1007/s00401-012-0958-8.
Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31:2927–35. https://doi.org/10.1200/JCO.2012.48.5052.
Shih DJH, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32:886–96. https://doi.org/10.1200/JCO.2013.50.9539.
Diao S, Gu C, Zhang H, Yu C. Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma. Oncol Lett. 2020;20:397. https://doi.org/10.3892/ol.2020.12260.
Grabovska Y, Mackay A, O’Hare P, et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat Commun. 2020;11:4324. https://doi.org/10.1038/s41467-020-18070-y.
Dang MT, Gonzalez MV, Gaonkar KS, et al. Macrophages in SHH subgroup medulloblastoma display dynamic heterogeneity that varies with treatment modality. Cell Rep. 2021;34:108917. https://doi.org/10.1016/j.celrep.2021.108917.
Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68. https://doi.org/10.1186/s13046-022-02272-x.
Chien F, Michaud ME, Bakhtiari M, et al. Medulloblastoma spatial transcriptomics reveals tumor microenvironment heterogeneity with high-density progenitor cell regions correlating with high-risk disease. BioRxiv. 2024. https://doi.org/10.1101/2024.06.25.600684.
Yuan L, Zhang H, Liu J, et al. STAT3 is required for Smo-dependent signaling and mediates Smo-targeted treatment resistance and tumorigenesis in Shh medulloblastoma. Mol Oncol. 2022;16:1009–25. https://doi.org/10.1002/1878-0261.13097.
Pham CD, Flores C, Yang C, et al. Differential immune microenvironments and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma. Clin Cancer Res. 2016;22:582–95. https://doi.org/10.1158/1078-0432.CCR-15-0713.
Marques RF, Moreno DA, da Silva L, et al. Digital expression profile of immune checkpoint genes in medulloblastomas identifies CD24 and CD276 as putative immunotherapy targets. Front Immunol. 2023;14:1062856. https://doi.org/10.3389/fimmu.2023.1062856.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524:3865–95. https://doi.org/10.1002/cne.24040.
Cheng Y, Franco-Barraza J, Wang Y, et al. Sustained hedgehog signaling in medulloblastoma tumoroids is attributed to stromal astrocytes and astrocyte-derived extracellular matrix. Lab Invest. 2020;100:1208–22. https://doi.org/10.1038/s41374-020-0443-2.
Gong B, Guo D, Zheng C, et al. Complement C3a activates astrocytes to promote medulloblastoma progression through TNF-α. J Neuroinflammation. 2022;19:159. https://doi.org/10.1186/s12974-022-02516-9.
Liu H, Sun Y, O’Brien JA, et al. Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro Oncol. 2020;22:625–38. https://doi.org/10.1093/neuonc/noz214.
Liu Y, Yuelling LW, Wang Y, et al. Astrocytes promote medulloblastoma progression through hedgehog secretion. Cancer Res. 2017;77:6692–703. https://doi.org/10.1158/0008-5472.CAN-17-1463.
Buonfiglioli A, Hambardzumyan D. Macrophages and microglia: the cerberus of glioblastoma. Acta Neuropathol Commun. 2021;9:54. https://doi.org/10.1186/s40478-021-01156-z.
Yao M, Ventura PB, Jiang Y, et al. Astrocytic trans-differentiation completes a multicellular paracrine feedback loop required for medulloblastoma tumor growth. Cell. 2020;180:502-520.e19. https://doi.org/10.1016/j.cell.2019.12.024.
Linke F, Aldighieri M, Lourdusamy A, et al. 3D hydrogels reveal medulloblastoma subgroup differences and identify extracellular matrix subtypes that predict patient outcome. J Pathol. 2021;253:326–38. https://doi.org/10.1002/path.5591.
Egeblad M, Shen H-CJ, Behonick DJ, et al. Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn. 2007;236:1683–93. https://doi.org/10.1002/dvdy.21159.
Jackson HK, Mitoko C, Linke F, et al. Extracellular vesicles potentiate medulloblastoma metastasis in an EMMPRIN and MMP-2 dependent manner. Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15092601.
Spyrou A, Kundu S, Haseeb L, et al. Inhibition of heparanase in pediatric brain tumor cells attenuates their proliferation, invasive capacity, and in vivo tumor growth. Mol Cancer Ther. 2017;16:1705–16. https://doi.org/10.1158/1535-7163.MCT-16-0900.
Northcott PA, Hielscher T, Dubuc A, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122:231–40. https://doi.org/10.1007/s00401-011-0846-7.
Mühlisch J, Bajanowski T, Rickert CH, et al. Frequent but borderline methylation of p16 (INK4a) and TIMP3 in medulloblastoma and sPNET revealed by quantitative analyses. J Neurooncol. 2007;83:17–29. https://doi.org/10.1007/s11060-006-9309-8.
Rustenhoven J, Kipnis J. Brain borders at the central stage of neuroimmunology. Nature. 2022;612:417–29. https://doi.org/10.1038/s41586-022-05474-7.
Rustenhoven J, Pavlou G, Storck SE, et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J Exp Med. 2023;220. https://doi.org/10.1084/jem.20221929.
Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91. https://doi.org/10.1038/s41586-018-0368-8.
Garzia L, Kijima N, Morrissy AS, et al. A hematogenous route for medulloblastoma leptomeningeal metastases. Cell. 2018;172:1050-1062.e14. https://doi.org/10.1016/j.cell.2018.01.038.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. https://doi.org/10.1101/cshperspect.a020412.
Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29:508–22. https://doi.org/10.1016/j.ccell.2016.03.002.
Benz F, Wichitnaowarat V, Lehmann M, et al. Low wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. eLife. 2019;8. https://doi.org/10.7554/eLife.43818.
Ward SA, Warrington NM, Taylor S, et al. Reprogramming medulloblastoma-propagating cells by a combined antagonism of sonic hedgehog and CXCR4. Cancer Res. 2017;77:1416–26. https://doi.org/10.1158/0008-5472.CAN-16-0847.
Yuan L, Zhang H, Liu J, et al. Growth factor receptor-Src-mediated suppression of GRK6 dysregulates CXCR4 signaling and promotes medulloblastoma migration. Mol Cancer. 2013;12:18. https://doi.org/10.1186/1476-4598-12-18.
Thompson EM, Keir ST, Venkatraman T, et al. The role of angiogenesis in Group 3 medulloblastoma pathogenesis and survival. Neuro Oncol. 2017;19:1217–27. https://doi.org/10.1093/neuonc/nox033.
Baudino TA, McKay C, Pendeville-Samain H, et al. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–43. https://doi.org/10.1101/gad.1024602.
Gold MP, Ong W, Masteller AM, et al. Developmental basis of SHH medulloblastoma heterogeneity. Nat Commun. 2024;15:270. https://doi.org/10.1038/s41467-023-44300-0.
Okonechnikov K, Joshi P, Sepp M, et al. Mapping pediatric brain tumors to their origins in the developing cerebellum. Neuro Oncol. 2023;25:1895–909. https://doi.org/10.1093/neuonc/noad124.
Griffith JI, Rathi S, Zhang W, et al. Addressing BBB heterogeneity: a new paradigm for drug delivery to brain tumors. Pharmaceutics. 2020;12. https://doi.org/10.3390/pharmaceutics12121205.
Welby JP, Kaptzan T, Wohl A, et al. Current murine models and new developments in H3K27M diffuse midline gliomas. Front Oncol. 2019;9:92. https://doi.org/10.3389/fonc.2019.00092.
Mohamed MR, Rich DQ, Seplaki C, et al. Primary treatment modification and treatment tolerability among older chemotherapy recipients with advanced cancer. JAMA Netw Open. 2024;7:e2356106. https://doi.org/10.1001/jamanetworkopen.2023.56106.
Barry E, Walsh JA, Weinrich SL, et al. Navigating the regulatory landscape to develop pediatric oncology drugs: expert opinion recommendations. Paediatr Drugs. 2021;23:381–94. https://doi.org/10.1007/s40272-021-00455-1.
Pearson AD, Mueller S, Filbin MG, et al. Paediatric strategy forum for medicinal product development in diffuse midline gliomas in children and adolescents ACCELERATE in collaboration with the European medicines agency with participation of the food and drug administration. Eur J Cancer. 2025;217:115230. https://doi.org/10.1016/j.ejca.2025.115230.
Stockwell, C. Figure 2: created in BioRender. 2025. https://www.BioRender.com/l77x596. Accessed 12 Mar 2025.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. https://doi.org/10.1126/science.aar4060.
Lim SH, Beers SA, Al-Shamkhani A, Cragg MS. Agonist antibodies for cancer immunotherapy: history, hopes, and challenges. Clin Cancer Res. 2024;30:1712–23. https://doi.org/10.1158/1078-0432.CCR-23-1014.
Fu Y, Tang R, Zhao X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol. 2023;14:1218082. https://doi.org/10.3389/fimmu.2023.1218082.
Foster JB, Madsen PJ, Hegde M, et al. Immunotherapy for pediatric brain tumors: past and present. Neuro Oncol. 2019;21:1226–38. https://doi.org/10.1093/neuonc/noz077.
Gorsi HS, Malicki DM, Barsan V, et al. Nivolumab in the treatment of recurrent or refractory pediatric brain tumors: a single institutional experience. J Pediatr Hematol Oncol. 2019;41:e235–41. https://doi.org/10.1097/MPH.0000000000001339.
Blumenthal DT, Yalon M, Vainer GW, et al. Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors. J Neurooncol. 2016;129:453–60. https://doi.org/10.1007/s11060-016-2190-1.
Penas-Prado M, Yuan Y, Wall K, et al. Ctim-32. immune checkpoint inhibitor nivolumab in people with recurrent select rare cns cancers: results of interim analysis in a heavily pretreated cohort. Neuro Oncol. 2021;23:vi57–8. https://doi.org/10.1093/neuonc/noab196.224.
Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34:2206–11. https://doi.org/10.1200/JCO.2016.66.6552.
AlHarbi M, Ali Mobark N, AlMubarak L, et al. Durable response to nivolumab in a pediatric patient with refractory glioblastoma and constitutional biallelic mismatch repair deficiency. Oncologist. 2018;23:1401–6. https://doi.org/10.1634/theoncologist.2018-0163.
Das A, Sudhaman S, Morgenstern D, et al. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat Med. 2022;28:125–35. https://doi.org/10.1038/s41591-021-01581-6.
Cacciotti C, Choi J, Alexandrescu S, et al. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol. 2020;149:113–22. https://doi.org/10.1007/s11060-020-03578-6.
Kline C, Liu SJ, Duriseti S, et al. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol. 2018;140:629–38. https://doi.org/10.1007/s11060-018-2991-5.
Kline C, Felton E, Allen IE, et al. Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol. 2018;137:103–10. https://doi.org/10.1007/s11060-017-2701-8.
Ausejo-Mauleon I, Labiano S, de la Nava D, et al. TIM-3 blockade in diffuse intrinsic pontine glioma models promotes tumor regression and antitumor immune memory. Cancer Cell. 2023;41:1911-1926.e8. https://doi.org/10.1016/j.ccell.2023.09.001.
Tan I-L, Arifa RDN, Rallapalli H, et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene. 2021;40:396–407. https://doi.org/10.1038/s41388-020-01536-0.
Cachia D, Eskandari R, McDonald DG, et al. Low-dose radiation followed by on-target inhibition of Galectin-3 in combination with anti-4–1BB monoclonal antibody regulates immune responses in Group 3 and Group 4 medulloblastoma mouse model (P11–13.001). In: Wednesday, April 26. Lippincott Williams & Wilkins. 2023. p. 3857. https://doi.org/10.1212/WNL.0000000000203573.
Johnson TS, Mcgaha T, Munn DH. Chemo-immunotherapy: role of indoleamine 2,3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment. Adv Exp Med Biol. 2017;1036:91–104. https://doi.org/10.1007/978-3-319-67577-0_7.
Sharma MD, Pacholczyk R, Shi H, et al. Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity. Immunity. 2021;54:2354-2371.e8. https://doi.org/10.1016/j.immuni.2021.09.005.
Johnson TS, MacDonald TJ, Pacholczyk R, et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: a first-in-children phase 1 trial. Neuro Oncol. 2023. https://doi.org/10.1093/neuonc/noad174.
Schuelke MR, Gundelach JH, Coffey M, et al. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neurooncol Adv. 2022;4:vdac085. https://doi.org/10.1093/noajnl/vdac085.
Kumar A, Taghi Khani A, Sanchez Ortiz A, Swaminathan S. GM-CSF: a double-edged sword in cancer immunotherapy. Front Immunol. 2022;13:901277. https://doi.org/10.3389/fimmu.2022.901277.
Ahn J, Shin C, Kim YS, et al. Cytomegalovirus-specific immunotherapy for glioblastoma treatments. Brain Tumor Res Treat. 2022;10:135–43. https://doi.org/10.14791/btrt.2022.0010.
Thompson E, Ashley DM, Ayasoufi K, et al. Outcomes and immune response after peptide vaccination targeting human cytomegalovirus antigen pp65 in children and young adults with recurrent high-grade glioma and medulloblastoma. JCO. 2024;42:2039–2039. https://doi.org/10.1200/JCO.2024.42.16_suppl.2039.
Mueller S, Taitt JM, Villanueva-Meyer JE, et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest. 2020;130:6325–37. https://doi.org/10.1172/JCI140378.
Fenstermaker RA, Ciesielski MJ. Challenges in the development of a survivin vaccine (SurVaxM) for malignant glioma. Expert Rev Vaccines. 2014;13:377–85. https://doi.org/10.1586/14760584.2014.881255.
Fangusaro JR, Jiang Y, Holloway MP, et al. Survivin, Survivin-2B, and Survivin-deItaEx3 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome. Br J Cancer. 2005;92:359–65. https://doi.org/10.1038/sj.bjc.6602317.
Chastkofsky MI, Pituch KC, Katagi H, et al. Mesenchymal stem cells successfully deliver oncolytic virotherapy to diffuse intrinsic pontine glioma. Clin Cancer Res. 2021;27:1766–77. https://doi.org/10.1158/1078-0432.CCR-20-1499.
Ahluwalia MS, Reardon DA, Abad AP, et al. Phase iia study of survaxm plus adjuvant temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2023;41:1453–65. https://doi.org/10.1200/JCO.22.00996.
Fellner C. Vismodegib (erivedge) for advanced Basal cell carcinoma. P T. 2012;37:670–82.
Guo Y, Lee H, Fang Z, et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci Adv. 2021;7. https://doi.org/10.1126/sciadv.abf7390.
Guo Y, Lee H, Fang Z, et al. Epct-25. smo protein depletion in shh medulloblastomas using microbubble-enhanced ultrasound and sirna loaded cationic nanoparticles. Neuro Oncol. 2021;23:i52–i52. https://doi.org/10.1093/neuonc/noab090.211.
Kim J, Dey A, Malhotra A, et al. Engineered biomimetic nanoparticle for dual targeting of the cancer stem-like cell population in sonic hedgehog medulloblastoma. Proc Natl Acad Sci USA. 2020;117:24205–12. https://doi.org/10.1073/pnas.1911229117.
MacDonald TJ, Liu J, Yu B, et al. Liposome-imipramine blue inhibits sonic hedgehog medulloblastoma in vivo. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13061220.
Tomaszewski WH, Waibl-Polania J, Chakraborty M, et al. Neuronal CaMKK2 promotes immunosuppression and checkpoint blockade resistance in glioblastoma. Nat Commun. 2022;13:6483. https://doi.org/10.1038/s41467-022-34175-y.
Dang N-N, Li X-B, Zhang M, et al. NLGN3 upregulates expression of ADAM10 to promote the cleavage of NLGN3 via activating the LYN pathway in human gliomas. Front Cell Dev Biol. 2021;9:662763. https://doi.org/10.3389/fcell.2021.662763.
Barron T, Mehta V, Woo P, Monje M. Hgg-04. targeting gabaergic neuron-glioma synapses in diffuse intrinsic pontine glioma (dipg) through anti-epileptic drug repurposing. Neuro Oncol. 2021;23:i17–8. https://doi.org/10.1093/neuonc/noab090.070.
Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. https://doi.org/10.1016/j.jconrel.2017.12.015.
Johnsen KB, Burkhart A, Melander F, et al. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci Rep. 2017;7:10396. https://doi.org/10.1038/s41598-017-11220-1.
Ye Z, Gastfriend BD, Umlauf BJ, et al. Antibody-targeted liposomes for enhanced targeting of the blood-brain barrier. Pharm Res. 2022;39:1523–34. https://doi.org/10.1007/s11095-022-03186-1.
Xu H, Li C, Wei Y, et al. Angiopep-2-modified calcium arsenite-loaded liposomes for targeted and pH-responsive delivery for anti-glioma therapy. Biochem Biophys Res Commun. 2021;551:14–20. https://doi.org/10.1016/j.bbrc.2021.02.138.
Lakkadwala S, Dos Santos RB, Sun C, Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Control Release. 2019;307:247–60. https://doi.org/10.1016/j.jconrel.2019.06.033.
Lam FC, Morton SW, Wyckoff J, et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9:1991. https://doi.org/10.1038/s41467-018-04315-4.
Liaw K, Zhang F, Mangraviti A, et al. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng Transl Med. 2020;5:e10160. https://doi.org/10.1002/btm2.10160.
Wang K, Zhang X, Liu Y, et al. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials. 2014;35:8735–47. https://doi.org/10.1016/j.biomaterials.2014.06.042.
Li Y, He H, Jia X, et al. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33:3899–908. https://doi.org/10.1016/j.biomaterials.2012.02.004.
Shan S, Chen J, Sun Y, et al. Functionalized macrophage exosomes with panobinostat and PPM1D-siRNA for diffuse intrinsic pontine gliomas therapy. Adv Sci (Weinh). 2022;9:e2200353. https://doi.org/10.1002/advs.202200353.
Hwang D, Dismuke T, Tikunov A, et al. Poly(2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity. Nanomedicine. 2021;32:102345. https://doi.org/10.1016/j.nano.2020.102345.
Tosi U, Souweidane M. Convection enhanced delivery for diffuse intrinsic pontine glioma: review of a single institution experience. Pharmaceutics. 2020;12. https://doi.org/10.3390/pharmaceutics12070660.
Souweidane MM, Kramer K, Pandit-Taskar N, et al. Phase 1 dose-escalation trial using convection-enhanced delivery of radiolabeled monoclonal antibody for diffuse intrinsic pontine glioma following external radiation therapy. JCO. 2021;39:2010–2010. https://doi.org/10.1200/JCO.2021.39.15_suppl.2010.
Szalontay L, CreveCoeur T, Neira J, et al. Surg-07. a phase i study examining the feasibility of intermittent convection-enhanced delivery (ced) of mtx110 for the treatment of children with newly diagnosed diffuse midline gliomas. Neuro Oncol. 2024;26. https://doi.org/10.1093/neuonc/noae064.648.
Mueller S, Kline C, Stoller S, et al. PNOC015: Repeated convection-enhanced delivery of MTX110 (aqueous panobinostat) in children with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol. 2023;25:2074–86. https://doi.org/10.1093/neuonc/noad105.
Sandberg DI, Kharas N, Yu B, et al. High-dose MTX110 (soluble panobinostat) safely administered into the fourth ventricle in a nonhuman primate model. J Neurosurg Pediatr. 2020;26:127–35. https://doi.org/10.3171/2020.2.PEDS19786.
Vitanza NA, Ronsley R, Choe M, et al. Locoregional CAR T cells for children with CNS tumors: Clinical procedure and catheter safety. Neoplasia. 2023;36:100870. https://doi.org/10.1016/j.neo.2022.100870.
Vitanza NA, Wilson AL, Huang W, et al. Intraventricular B7–H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2023;13:114–31. https://doi.org/10.1158/2159-8290.CD-22-0750.
Stockwell, C. Figure 3: created in BioRender. 2025. https://www.BioRender.com/c92n937. Accessed 12 Mar 2025.
Sajesh BV, On NH, Omar R, et al. Validation of cadherin HAV6 peptide in the transient modulation of the blood-brain barrier for the treatment of brain tumors. Pharmaceutics. 2019;11. https://doi.org/10.3390/pharmaceutics11090481.
Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother. 2015;15:477–91. https://doi.org/10.1586/14737175.2015.1028369.
Milos P, Haj-Hosseini N, Hillman J, Wårdell K. 5-ALA fluorescence in randomly selected pediatric brain tumors assessed by spectroscopy and surgical microscope. Acta Neurochir (Wien). 2023;165:71–81. https://doi.org/10.1007/s00701-022-05360-1.
Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77:663–73. https://doi.org/10.1227/NEU.0000000000000929.
Syed HR, Packer RJ, Fonseca A, et al. Trls-17. interim clinical, pharmacokinetic, and technical report of a phase 2 study of sonodynamic therapy (sdt) using sonala-001 together with mr-guided low-intensity focused ultrasound (mrgfus) in children with diffuse intrinsic pontine glioma (dipg). Neuro Oncol. 2024;26. https://doi.org/10.1093/neuonc/noae064.170.
Bai R-Y, Staedtke V, Rudin CM, et al. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol. 2015;17:545–54. https://doi.org/10.1093/neuonc/nou234.
Chan TSY, Picard D, Hawkins CE, et al. Thrombospondin-1 mimetics are promising novel therapeutics for MYC-associated medulloblastoma. Neurooncol Adv. 2021;3:vdab002. https://doi.org/10.1093/noajnl/vdab002.
Liang Z, Brooks J, Willard M, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–22. https://doi.org/10.1016/j.bbrc.2007.05.182.
Peyrl A, Chocholous M, Sabel M, et al. Sustained survival benefit in recurrent medulloblastoma by a metronomic antiangiogenic regimen: a nonrandomized controlled trial. JAMA Oncol. 2023;9:1688–95. https://doi.org/10.1001/jamaoncol.2023.4437.
Karachi A, Dastmalchi F, Nazarian S, et al. Optimizing T cell-based therapy for glioblastoma. Front Immunol. 2021;12:705580. https://doi.org/10.3389/fimmu.2021.705580.
Nehama D, Woodell AS, Maingi SM, et al. Cell-based therapies for glioblastoma: promising tools against tumor heterogeneity. Neuro Oncol. 2023;25:1551–62. https://doi.org/10.1093/neuonc/noad092.
Zhang X, Zhu L, Zhang H, et al. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front Immunol. 2022;13:927153. https://doi.org/10.3389/fimmu.2022.927153.
Patterson JD, Henson JC, Breese RO, et al. CAR T cell therapy for pediatric brain tumors. Front Oncol. 2020;10:1582. https://doi.org/10.3389/fonc.2020.01582.
Thomas P, Galopin N, Bonérandi E, et al. CAR T cell therapy’s potential for pediatric brain tumors. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13215445.
Wu W-T, Lin W-Y, Chen Y-W, et al. New era of immunotherapy in pediatric brain tumors: chimeric antigen receptor T-cell therapy. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22052404.
Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3–K27M+ diffuse midline gliomas. Nat Med. 2018;24:572–9. https://doi.org/10.1038/s41591-018-0006-x.
Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–41. https://doi.org/10.1038/s41586-022-04489-4.
Monje M, Mahdi J, Majzner R, et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature. 2025;637:708–15. https://doi.org/10.1038/s41586-024-08171-9.
Vitanza NA, Ronsley R, Huang W, et al. Trls-01. intraventricular b7–h3 car t cells for diffuse intrinsic pontine glioma: safety and efficacy report from the completed phase 1 trial brainchild-03. Neuro Oncol. 2024;26. https://doi.org/10.1093/neuonc/noae064.154.
Vitanza NA, Ronsley R, Choe M, et al. Intracerebroventricular B7–H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial. Nat Med. 2025. https://doi.org/10.1038/s41591-024-03451-3.
Zuo P, Li Y, He C, et al. Anti-tumor efficacy of anti-GD2 CAR NK-92 cells in diffuse intrinsic pontine gliomas. Front Immunol. 2023;14:1145706. https://doi.org/10.3389/fimmu.2023.1145706.
Bagó JR, Alfonso-Pecchio A, Okolie O, et al. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun. 2016;7:10593. https://doi.org/10.1038/ncomms10593.
Okolie O, Irvin DM, Bago JR, et al. Intra-cavity stem cell therapy inhibits tumor progression in a novel murine model of medulloblastoma surgical resection. PLoS ONE. 2018;13:e0198596. https://doi.org/10.1371/journal.pone.0198596.
Lassaletta A, Vazquez-Gomez F, Rubio A, et al. Dipg-81. phase ib clinical trial assessing safety, tolerability, and preliminary efficacy of alocelyvir (allogeneic mesenchymal cells + oncolytic adenovirus) combined with radiotherapy in pediatric patients with newly diagnosed diffuse intrinsic pontine glioma (dipg). Neuro Oncol. 2024;26. https://doi.org/10.1093/neuonc/noae064.134.
Canella A, Nazzaro M, Rajendran S, et al. Genetically modified IL2 bone-marrow-derived myeloid cells reprogram the glioma immunosuppressive tumor microenvironment. Cell Rep. 2023;42:112891. https://doi.org/10.1016/j.celrep.2023.112891.
Foster JB, Alonso MM, Sayour E, et al. Translational considerations for immunotherapy clinical trials in pediatric neuro-oncology. Neoplasia. 2023;42:100909. https://doi.org/10.1016/j.neo.2023.100909.
Johung TB, Monje M. Diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr Neuropharmacol. 2017;15:88–97. https://doi.org/10.2174/1570159x14666160509123229.
Mann B, Zhang X, Bell N, et al. A living ex vivo platform for functional, personalized brain cancer diagnosis. Cell Rep Med. 2023;4:101042. https://doi.org/10.1016/j.xcrm.2023.101042.
Satterlee AB, Dunn DE, Valdivia A, et al. Spatiotemporal analysis of induced neural stem cell therapy to overcome advanced glioblastoma recurrence. Mol Ther Oncolytics. 2022;26:49–62. https://doi.org/10.1016/j.omto.2022.06.004.
Satterlee AB, Dunn DE, Lo DC, et al. Tumoricidal stem cell therapy enables killing in novel hybrid models of heterogeneous glioblastoma. Neuro Oncol. 2019;21:1552–64. https://doi.org/10.1093/neuonc/noz138.
Eichmüller OL, Knoblich JA. Human cerebral organoids - a new tool for clinical neurology research. Nat Rev Neurol. 2022;18:661–80. https://doi.org/10.1038/s41582-022-00723-9.
Sun N, Meng X, Liu Y, et al. Applications of brain organoids in neurodevelopment and neurological diseases. J Biomed Sci. 2021;28:30. https://doi.org/10.1186/s12929-021-00728-4.
Mariappan A, Goranci-Buzhala G, Ricci-Vitiani L, et al. Trends and challenges in modeling glioma using 3D human brain organoids. Cell Death Differ. 2021;28:15–23. https://doi.org/10.1038/s41418-020-00679-7.
Proietto M, Crippa M, Damiani C, et al. Tumor heterogeneity: preclinical models, emerging technologies, and future applications. Front Oncol. 2023;13:1164535. https://doi.org/10.3389/fonc.2023.1164535.
Pan S, Ye D, Yue Y, et al. Leptomeningeal disease and tumor dissemination in a murine diffuse intrinsic pontine glioma model: implications for the study of the tumor-cerebrospinal fluid-ependymal microenvironment. Neurooncol Adv. 2022;4:vdac059. https://doi.org/10.1093/noajnl/vdac059.
Weidenhammer LB, Liu HQ, Luo L, et al. Inducing primary brainstem gliomas in genetically engineered mice using RCAS/TVA retroviruses and Cre/loxP recombination. STAR Protocols. 2023;4:102094. https://doi.org/10.1016/j.xpro.2023.102094.
Roussel MF, Stripay JL. Modeling pediatric medulloblastoma. Brain Pathol. 2020;30:703–12. https://doi.org/10.1111/bpa.12803.
Becher OJ, Holland EC. Evidence for and against regional differences in neural stem and progenitor cells of the CNS. Genes Dev. 2010;24:2233–8. https://doi.org/10.1101/gad.1988010.
Misuraca KL, Hu G, Barton KL, et al. A novel mouse model of diffuse intrinsic pontine glioma initiated in Pax3-expressing cells. Neoplasia. 2016;18:60–70. https://doi.org/10.1016/j.neo.2015.12.002.
Yuan B, Wang G, Tang X, et al. Immunotherapy of glioblastoma: recent advances and future prospects. Hum Vaccin Immunother. 2022;18:2055417. https://doi.org/10.1080/21645515.2022.2055417.
Lim C, Dismuke T, Malawsky D, et al. Enhancing CDK4/6 inhibitor therapy for medulloblastoma using nanoparticle delivery and scRNA-seq-guided combination with sapanisertib. Sci Adv. 2022;8:eabl5838. https://doi.org/10.1126/sciadv.abl5838.
Kawauchi D, Robinson G, Uziel T, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21:168–80. https://doi.org/10.1016/j.ccr.2011.12.023.
Heller A, Du F, Liu Y, et al. Generating a mouse model for relapsed Sonic Hedgehog medulloblastoma. STAR Protocols. 2023;4:102234. https://doi.org/10.1016/j.xpro.2023.102234.
Mendez-Gomez HR, DeVries A, Castillo P, et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell. 2024;187:2521-2535.e21. https://doi.org/10.1016/j.cell.2024.04.003.
Acknowledgements
The authors would like to thank Dr. Leaf Huang for his mentorship and feedback on the early stages of this article. Figures were created in Biorender.
Funding
This work was supported by National Institutes of Health T32 funding from the Program in Translational Medicine at the University of North Carolina at Chapel Hill (NIH T32GM122741), National Center for Advancing Translational Sciences (K12TR004416), National Institutes of Health (U01TR003715), and by the Isabella Santos Foundation TORCH Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of this work. The first draft of the manuscript was written by C.A.S. and all authors contributed to the writing and revision process. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approvals were required for this review and no human subjects were involved.
Competing interests
We affirm that our manuscript has not been submitted for publication elsewhere, and there are no undeclared competing financial interest. S.H., and A.B.S. have submitted a patent application based on the OBSC platform mentioned in this work. C.A.S, M.T. and D.E.K declare no competing interest.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stockwell, C.A., Thang, M., Kram, D.E. et al. Therapeutic approaches for targeting the pediatric brain tumor microenvironment. Drug Deliv. and Transl. Res. (2025). https://doi.org/10.1007/s13346-025-01839-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-025-01839-3