Abstract
Biodegradable nanocarriers possess enormous potential for use as drug delivery systems that can accomplish controlled and targeted drug release, and a wide range of nanosystems have been reported for the treatment and/or diagnosis of various diseases and disorders. Of the various nanocarriers currently available, liposomes and polymer nanoparticles have been extensively studied and some formulations have already reached the market. However, a combination of properties to create a single hybrid system can give these carriers significant advantages, such as improvement in encapsulation efficacy, higher stability, and active targeting towards specific cells or tissues, over lipid or polymer-based platforms. To this aim, this work presents the formulation of poly(lactic-co-glycolic) acid (PLGA) nanoparticles in the presence of a hyaluronic acid (HA)-phospholipid conjugate (HA-DPPE), which was used to anchor HA onto the nanoparticle surface and therefore create an actively targeted hybrid nanosystem. Furthermore, ionic interactions have been proposed for drug encapsulation, leading us to select the free base form of pentamidine (PTM-B) as the model drug. We herein report the preparation of hybrid nanocarriers that were loaded via ion-pairing between the negatively charged PLGA and HA and the positively charged PTM-B, demonstrating an improved loading capacity compared to PLGA-based nanoparticles. The nanocarriers displayed a size of below 150 nm, a negative zeta potential of -35 mV, a core-shell internal arrangement and high encapsulation efficiency (90%). Finally, the ability to be taken up and exert preferential and receptor-mediated cytotoxicity on cancer cells that overexpress the HA specific receptor (CD44) has been evaluated. Competition assays supported the hypothesis that PLGA/HA-DPPE nanoparticles deliver their cargo within cells in a CD44-dependent manner.
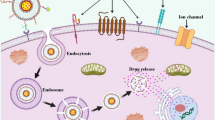
Graphical Abstract








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Additional data related to this paper may be requested from the authors.
References
Mitchell MJ, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Fan YN, et al. Progress in nanoparticle-based regulation of immune cells. Med Rev (Berl). 2023;3(2):152–79.
Zhang P, et al. Nbtxr3 radiotherapy-activated functionalized hafnium oxide nanoparticles show efficient antitumor effects across a large panel of human cancer models. Int J Nanomed. 2021;16:2761–73.
Guo B, et al. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater. 2023;35(22):e2212267.
Rao Z, et al. Iron-based metal-organic framework co-loaded with buthionine sulfoximine and oxaliplatin for enhanced cancer chemo-ferrotherapy via sustainable glutathione elimination. J Nanobiotechnol. 2023;21(1):265.
Tenchov R, et al. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–7015.
Park H, Otte A, Park K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J Control Release. 2022;342:53–65.
Liu Y, et al. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023;14(1):28.
Ta HT, et al. The effects of particle size, shape, density and flow characteristics on particle margination to vascular walls in cardiovascular diseases. Expert Opin Drug Deliv. 2018;15(1):33–45.
Li X, et al. Design of Smart Size-, Surface-, and Shape-Switching Nanoparticles to Improve Therapeutic Efficacy. Small. 2022;18(6):e2104632.
Gamble JF, et al. Morphological distribution mapping: Utilisation of modelling to integrate particle size and shape distributions. Int J Pharm. 2023;635:122743.
Sivadasan D, et al. Polymeric lipid hybrid nanoparticles (PLNs) as emerging drug delivery platform-a comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics. 2021;13(8):1291.
Jain S, et al. Lipid-polymer hybrid nanosystems: a rational fusion for advanced therapeutic delivery. J Funct Biomater. 2023;14(9):437.
He C, Lu J, Lin W. Hybrid nanoparticles for combination therapy of cancer. J Control Release. 2015;219:224–36.
Dehaini D, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29(16):10.1002.
Ferreira Soares DC, et al. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed Pharmacother. 2020;131:110695.
Dhiman N, et al. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 2021;9:580118.
Yun P, Devahastin S, Chiewchan N. Microstructures of encapsulates and their relations with encapsulation efficiency and controlled release of bioactive constituents: A review. Compr Rev Food Sci Food Saf. 2021;20(2):1768–99.
Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34.
Adams D, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21.
Schoenmaker L, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021;601:120586.
Jia Y, et al. Lipid nanoparticles optimized for targeting and release of nucleic acid. Adv Mater. 2024;36(4):2305300.
Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–63.
Hald Albertsen C, et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416.
Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl. 2018;92:1041–60.
Idrees H, et al. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomater (Basel). 2020;10(10):1970.
Khalili L, et al. Smart active-targeting of lipid-polymer hybrid nanoparticles for therapeutic applications: Recent advances and challenges. Int J Biol Macromol. 2022;213:166–94.
Ghitman J, et al. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater Des. 2020;193:108805.
Arpicco S, et al. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur J Pharm Biopharm. 2013;85(3 Pt A):373–80.
Zeng X, et al. pH-Responsive hyaluronic acid nanoparticles for enhanced triple negative breast cancer therapy. Int J Nanomedicine. 2022;17:1437–57.
de Paula MC, et al. The role of hyaluronic acid in the design and functionalization of nanoparticles for the treatment of colorectal cancer. Carbohydr Polym. 2023;320:121257.
Stella B, et al. Pentamidine-loaded lipid and polymer nanocarriers as tunable anticancer drug delivery systems. J Pharm Sci. 2020;109(3):1297–302.
Andreana I, et al. Selective delivery of pentamidine toward cancer cells by self-assembled nanoparticles. Int J Pharm. 2022;625: 122102.
Andreana I, et al. Nanotechnological approaches for pentamidine delivery. Drug Deliv Transl Res. 2022;12(8):1911–27.
Peretti E, et al. Strategies to obtain encapsulation and controlled release of pentamidine in mesoporous silica nanoparticles. Pharmaceutics. 2018;10(4):195.
Fessi H, et al. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–4.
Mandal TK, et al. Poly(D, L-lactide-co-glycolide) encapsulated poly(vinyl alcohol) hydrogel as a drug delivery system. Pharm Res. 2002;19(11):1713–9.
Doucet M, et al. SasView version 5.0.3. Zenodo. 2020. https://doi.org/10.5281/zenodo.3930098.
Franze S, et al. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int J Pharm. 2018;535(1–2):333–9.
Cannito S, et al. Hyaluronated and PEGylated liposomes as a potential drug-delivery strategy to specifically target liver cancer and inflammatory cells. Molecules. 2022;27(3):1062.
Franze S, et al. Rationalizing the design of hyaluronic acid-decorated liposomes for targeting epidermal layers: a combination of molecular dynamics and experimental evidence. Mol Pharm. 2021;18(11):3979–89.
Pandolfi L, et al. Liposomes loaded with everolimus and coated with hyaluronic acid: a promising approach for lung fibrosis. Int J Mol Sci. 2021;22(14):7743.
Andreana I, et al. Freeze drying of polymer nanoparticles and liposomes exploiting different saccharide-based approaches. Mater (Basel). 2023;16(3):1212.
Camara CI, et al. Hyaluronic acid-dexamethasone nanoparticles for local adjunct therapy of lung inflammation. Int J Mol Sci. 2021;22(19):10480.
Di Cola E, et al. Novel O/W nanoemulsions for nasal administration: Structural hints in the selection of performing vehicles with enhanced mucopenetration. Colloids Surf B Biointerfaces. 2019;183:110439.
d’Angelo I, et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: development and fate upon in vitro deposition on the human epithelial airway barrier. J Aerosol Med Pulm Drug Deliv. 2018;31(3):170–81.
Clementino AR, et al. Structure and fate of nanoparticles designed for the nasal delivery of poorly soluble drugs. Mol Pharm. 2021;18(8):3132–46.
Li W, et al. Hyaluronic acid ion-pairing nanoparticles for targeted tumor therapy. J Control Release. 2016;225:170–82.
Chang G, et al. CD44 targets Na(+)/H(+) exchanger 1 to mediate MDA-MB-231 cells’ metastasis via the regulation of ERK1/2. Br J Cancer. 2014;110(4):916–27.
Corsetto PA, et al. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011;10:73.
Cano ME, et al. Synthesis of defined oligohyaluronates-decorated liposomes and interaction with lung cancer cells. Carbohydr Polym. 2020;248: 116798.
Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8(9):957.
Muley H, et al. Drug uptake-based chemoresistance in breast cancer treatment. Biochem Pharmacol. 2020;177:113959.
Wang L, et al. Gold nanomaterial system that enables dual photothermal and chemotherapy for breast cancer. Pharmaceutics. 2023;15(9):2198.
Mirzaei S, et al. Dual-targeted delivery system using hollow silica nanoparticles with H(+)-triggered bubble generating characteristic coated with hyaluronic acid and AS1411 for cancer therapy. Drug Dev Ind Pharm. 2023;49(10):648-57.
Abduh MS. Anticancer analysis of CD44 targeted cyclosporine loaded thiolated chitosan nanoformulations for sustained release in triple-negative breast cancer. Int J Nanomedicine. 2023;18:5713–32.
Spini A, et al. Repurposing of drugs for triple negative breast cancer: an overview. Ecancermedicalscience. 2020;14:1071.
Acknowledgements
The authors thank ESRF for financial support and beamtime (https://doi.org/10.15151/ESRF-ES-1351189712), ID02 staff for technical support and PSCM facility (Grenoble) for allowing on-site sample preparation. E.D.F. thanks BIOMETRA Dept. for partial support (PSR2021_DEL_FAVERO). This work benefited from the use of the SasView application.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Chiara Riganti, Silvia Arpicco, Barbara Stella; Methodology: Elena Del Favero, Chiara Riganti, Silvia Arpicco, Barbara Stella; Formal analysis and investigation: Ilaria Andreana, Marta Chiapasco, Valeria Bincoletto, Sabrina Digiovanni, Maela Manzoli, Caterina Ricci, Elena Del Favero; Writing - original draft preparation: Ilaria Andreana; Writing - review and editing: Sabrina Digiovanni, Maela Manzoli, Caterina Ricci, Elena Del Favero, Chiara Riganti, Silvia Arpicco, Barbara Stella; Supervision: Barbara Stella.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the version to be published.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreana, I., Chiapasco, M., Bincoletto, V. et al. Targeting pentamidine towards CD44-overexpressing cells using hyaluronated lipid-polymer hybrid nanoparticles. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01617-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01617-7