Abstract
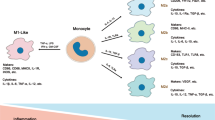
Extracellular vesicles (EVs) show promising potential to be used as therapeutics, disease biomarkers, and drug delivery vehicles. We aimed to modify EVs with miR-155 to modulate macrophage immune response that can be potentially used against infectious diseases. Primarily, we characterized T cells (EL-4) EVs by several standardized techniques and confirmed that the EVs could be used for experimental approaches. The bioactivities of the isolated EVs were confirmed by the uptake assessment, and the results showed that target cells can successfully uptake EVs. To standardize the loading protocol by electroporation for effective biological functionality, we chose fluorescently labelled miR-155 mimics because of its important roles in the immune regulations to upload them into EVs. The loading procedure showed that the dosage of 1 µg of miRNA mimics can be efficiently loaded to the EVs at 100 V, further confirmed by flow cytometry. The functional assay by incubating these modified EVs (mEVs) with in vitro cultured cells led to an increased abundance of miR-155 and decreased the expressions of its target genes such as TSHZ3, Jarid2, ZFP652, and WWC1. Further evaluation indicated that these mEVs induced M1-type macrophage polarization with increased TNF-α, IL-6, IL-1β, and iNOS expression. The bioavailability analysis revealed that mEVs could be detected in tissues of the livers. Overall, our study demonstrated that EVs can be engineered with miR-155 of interest to modulate the immune response that may have implications against infectious diseases.
Graphical abstract





Similar content being viewed by others
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
References
Morelli AE, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66.
Villarroya-Beltri C, et al. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:-13.
Fader CM, et al. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta Mol Cell Res. 2009;1793(12):1901–16.
Rodrigues M, et al. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics. 2018;8(10):2709–21.
El-Andaloussi S, et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7(12):2112–26.
Kamerkar S, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503.
Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66.
de Abreu RC, et al. Exogenous loading of miRNAs into small extracellular vesicles. J Extracell Vesicles. 2021;10(10):e12111.
Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Kim MS, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–64.
Sato YT, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.
Bartel DP. Metazoan microRNAs. Cell. 2018;173(1):20–51.
Ranganath P. MicroRNA-155 and its role in malignant hematopoiesis. Biomark Insights. 2015;10:95–102.
Cardoso AL, et al. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135(1):73–88.
Giri BR, Mahato RI, Cheng G. Roles of microRNAs in T cell immunity: implications for strategy development against infectious diseases. Med Res Rev. 2019;39(2):706–32.
Zhang ZT, et al. Dexmedetomidine alleviates acute lung injury by promoting Tregs differentiation via activation of AMPK/SIRT1 pathway. Inflammopharmacology. 2023;31(1):423–38.
Li S, et al. Characterization of microRNA cargo of extracellular vesicles isolated from the plasma of Schistosoma japonicum-infected mice. Front Cell Infect Microbiol. 2022;12: 803242.
Deng Z, et al. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics. 2021;11(9):4351–62.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Watanabe S, et al. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129(7):2619–28.
Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617.
Villarroya-Beltri C, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980.
Torralba D, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018;9(1):2658.
Wahlgren J, et al. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS ONE. 2012;7(11):e49723.
Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2(1):282.
Cai Z, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188(12):5954–61.
Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6(2):103–6.
Ohno S, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Czernek L, Pęczek Ł, Düchler M. Small extracellular vesicles loaded with immunosuppressive mirnas leads to an inhibition of dendritic cell maturation. Arch Immunol Ther Exp. 2022;70(1):27.
Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23(4):236–50.
Meizlish ML, et al. Tissue homeostasis and inflammation. Annu Rev Immunol. 2021;39:557–81.
Kurowska-Stolarska M, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108(27):11193–8.
Jing W, et al. CRISPR/CAS9-mediated genome editing of miRNA-155 inhibits proinflammatory cytokine production by RAW264. 7 cells. BioMed Res Int. 2015:326042.
Kim H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci Rep. 2017;7(1):7591.
He M, et al. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPβ. Cell Mol Immunol. 2009;6(5):343–52.
Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor α1 (IL13Rα1). J Biol Chem. 2011;286(3):1786–94.
Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-β. J Biol Chem. 2010;285(53):41328–36.
Salvi V, et al. Cytokine targeting by miRNAs in autoimmune diseases. Front Immunol. 2019;10:15.
Escobar TM, et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity. 2014;40(6):865–79.
Caballero-Garrido E, et al. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35(36):12446–64.
Wang L, Cao QM. Long non-coding RNA XIST alleviates sepsis-induced acute kidney injury through inhibiting inflammation and cell apoptosis via regulating miR-155-5p/WWC1 axis. Kaohsiung J Med Sci. 2022;38(1):6–17.
Usman WM, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1):2359.
Zhang G, et al. Extracellular vesicles: natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles. 2020;10(2):e12030.
Acknowledgements
We thank Cheng lab members for constructive suggestion on the discussion.
Funding
This work was supported, in whole or in part, by the Key Program for International S&T Cooperation Projects of China (2021YFE0191600 to GC) and the National Natural Science Foundation of China (31950410564 to BG, 31672550 to GC).
Author information
Authors and Affiliations
Contributions
Bikash R. Giri: conceptualization, investigation, analysis, writing, funding acquisition; Li Shun: investigation, analysis; Cheng Guofeng: conceptualization, supervision, methodology, editing, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal experiments were performed in compliance with the institutional guidelines and by following the protocol approved by the Institutional Care and Use Committee of Tongji University School of Medicine, China (TJAA00621101).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giri, B.R., Li, S. & Cheng, G. Exogenous modification of EL-4 T cell extracellular vesicles with miR-155 induce macrophage into M1-type polarization. Drug Deliv. and Transl. Res. 14, 934–944 (2024). https://doi.org/10.1007/s13346-023-01442-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01442-4