Abstract
The host immune system possesses an intrinsic ability to target and kill cancer cells in a specific and adaptable manner that can be further enhanced by cancer immunotherapy, which aims to train the immune system to boost the antitumor immune response. Several different categories of cancer immunotherapy have emerged as new standard cancer therapies in the clinic, including cancer vaccines, immune checkpoint inhibitors, adoptive T cell therapy, and oncolytic virus therapy. Despite the remarkable survival benefit for a subset of patients, the low response rate and immunotoxicity remain the major challenges for current cancer immunotherapy. Over the last few decades, nanomedicine has been intensively investigated with great enthusiasm, leading to marked advancements in nanoparticle platforms and nanoengineering technology. Advances in nanomedicine and immunotherapy have also led to the emergence of a nascent research field of nano-immunotherapy, which aims to realize the full therapeutic potential of immunotherapy with the aid of nanomedicine. In particular, nanocarriers present an exciting opportunity in immuno-oncology to boost the activity, increase specificity, decrease toxicity, and sustain the antitumor efficacy of immunological agents by potentiating immunostimulatory activity and favorably modulating pharmacological properties. This review discusses the potential of nanocarriers for cancer immunotherapy and introduces preclinical studies designed to improve clinical cancer immunotherapy modalities using nanocarrier-based engineering approaches. It also discusses the potential of nanocarriers to address the challenges currently faced by immuno-oncology as well as the challenges for their translation to clinical applications.
Graphical abstract

Similar content being viewed by others
Introduction
Cancer presents considerable health risk with a significant mortality rate worldwide; according to American Cancer Society, around 1.9 million people were diagnosed with cancer in 2021 [1], and the cancer-associated mortality is expected to reach 22 million by the year of 2030 [2]. Conventional cancer treatment methods, such as surgery, chemotherapy, and radiotherapy, have limited efficacy, particularly against advanced cancers with metastasis [3, 4]. Since William B. Coley reported the first systemic study of immunotherapy using bacterial toxins for the treatment of sarcoma in 1891 [5], cancer immunotherapy has become a new treatment option for many different cancers [6, 7]. Cancer immunotherapy harnesses the host immune system by training immune cells to specifically recognize and kill cancer cells. The immune system can also confer long-term protection against tumor recurrence and metastasis via immunological memory. The remarkable clinical success of cancer immunotherapy has been highlighted by the durable survival benefit for a subset of patients, in some cases leading to complete tumor remission [8]. The groundbreaking achievement in patient outcome was recognized by Science as a “breakthrough of the year” in 2013 [9] and also acknowledged with a Noble Prize in Physiology or Medicine 2018, which was awarded to James Allison and Tasuku Honjo, who proposed immune checkpoint regulation for cancer treatment.
Although immunotherapy has emerged as a new standard pillar for cancer therapy, the challenges remain as it usually benefits only a small subset of patients and causes immunotoxicity, limiting its wide clinical applications [10]. Advances in nanomedicine and immunotherapy have led to the emergence of a nascent research field that aims to realize the full therapeutic potential of immunotherapy with the aid of nanoparticle platforms and nanoengineering technology. Recent preclinical studies have demonstrated the potential of nano-immunotherapy to address the challenges faced by current cancer immunotherapy. In this review, we introduce the basic principles of the antitumor immune response and the potential advantages of nanocarriers for cancer immunotherapy. We discuss how nanocarrier-based engineering approaches can improve clinical cancer immunotherapy modalities, including cancer vaccines, immune checkpoint inhibitors, adoptive T cell therapy, and oncolytic virus therapy. Finally, we discuss the potential of nanocarriers for cancer immunotherapy and the challenges in their clinical translation. We also argue that nano-immunotherapy presents a significant opportunity not only for nanomedicine but also for immunotherapy to fulfill their clinical impact that may lead to a new revolution in immuno-oncology.
Antitumor immune response
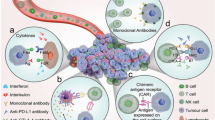
The immune system is composed of innate and adaptive immunity to orchestrate the host immune response, which confers the first line of defense against foreign substances in a rapid and non-specific manner by innate immunity, and induces the secondary defense mechanism of adaptive immunity that mounts a durable immune response against specific antigens [11]. The immune response can also be mounted against transformed or damaged cells of host origin, including viral and cancer cells [12], of the latter by a process known as tumor immune surveillance [13]. Innate immune cells specialized for antigen presentation are called professional antigen-presenting cells (APCs) and include dendritic cells (DCs) and macrophages. APCs can not only present tumor-associated antigens (TAAs) in the context of major histocompatibility complexes (MHCs) but also provide activation signals transmitted via co-stimulatory receptors such as Toll-like receptors for optimal priming of the adaptive immune response by T cells [14, 15]. Several intracellular molecules released from cancer cells, including high-mobility group box 1, heat-shock proteins, and adenosine triphosphate, have been shown to stimulate activation signals in APCs [16]. In addition, the class of MHC molecules determines the type of T cell response against specific antigens; antigens bound to MHC class I elicit CD8+ T cell responses, while MHC class II elicits CD4+ T cell responses [17, 18]. Particularly, CD8+ T cells play an indispensable role in the antitumor immune response by recognizing the TAA–MHC-I complex expressed on nascent cancer cells and subsequently killing them via direct cellular cytotoxic mechanisms; therefore, they are also called cytotoxic T lymphocytes (CTLs) [19]. Innate and adaptive immune cells also release immunostimulatory cytokines that can activate or augment the antitumor immune response in an autocrine and/or paracrine fashion [20,21,22]. The immunogenic destruction of cancer cells can promote the release of TAA and immunostimulatory intracellular molecules, which further potentiates the antitumor immune response by tumor immune surveillance, leading to the self-sustained cancer immunity cycle (Fig. 1) [23]. Besides, cancer cells have evolved to escape the host immune response through various intrinsic and extrinsic mechanisms, resulting in the failure of tumor immune surveillance to control their outgrowth. Cancer cells can induce mutations in antigenic proteins and downregulate and upregulate MHC-I molecules and immune checkpoint ligands to evade and thwart the antitumor activity of T cells, respectively [24]. In addition, cancer cells can render the tumor microenvironment immunosuppressive with the induction of immune checkpoint receptors on T cells; attraction and polarization of immunosuppressive immune cells, such as tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs); and the release of immunosuppressive cytokines, such as transforming growth factor (TGF)-β and interleukin (IL)-10 [25]. Therefore, successful cancer immunotherapy requires preferential regulation of innate and adaptive immune cells for effective tumor immune surveillance and modulation of their interaction with cancer cells in the immunosuppressive tumor microenvironment in favor of antitumor immunity.

Adapted from reference [23] with permission
The cancer–immunity cycle.
Immunotherapy approaches using nanocarriers
Immunotherapy has become a new treatment option for many tumors. The representative categories of cancer immunotherapy that have progressed into the clinic include cancer vaccines, immune checkpoint inhibitors, adoptive T cell therapy, and oncolytic virus therapy, which have several marketed products approved by the Food and Drug Administration (FDA) for various tumor indications (Table 1). However, they remain to be improved in terms of the response rate and immunotoxicity to be more generally applicable to the patients. Nano-immunotherapy aims to realize the full therapeutic potential of immunotherapy with the aid of nanoparticle platforms and nanoengineering technology and, therefore, to address the challenges faced by current cancer immunotherapy [9]. In particular, nanocarriers have been demonstrated to boost the activity of immunostimulatory agents in a safe and effective manner in many preclinical studies, suggesting their potential in immuno-oncology (Table 2). In the following sections, we introduce preclinical studies designed to improve clinical cancer immunotherapy modalities using nanocarrier-based engineering approaches.
Nanocarriers for cancer immunotherapy
Nanocarriers offer several advantages as delivery platforms for biological drugs. The high surface area and surface-to-volume ratio render efficient surface loading and interparticle entrapment of various cargos, from small molecules to large proteins and nucleic acids [26]. It also allows effective engagement with the cell membrane to promote subsequent cellular uptake, while the relatively large nano-size blocks efflux transport from the cells, leading to robust uptake and accumulation in the cells [27]. In addition, nanoparticle formulation can improve the solubility, in vivo stability, and systemic circulation and biodistribution of payload drugs in the body [28]. One notable example is the delivery of nucleic acids that are typically prone to degradation by serum enzymes and exhibit a poor biodistribution profile with short circulation and deficient cellular uptake in vivo due to their unfavorable pharmacological properties [29]. Nanocarriers have been shown to protect against enzymatic degradation, prolong in vivo circulation, and improve target delivery and cellular uptake of nucleic acids for their efficient transcriptional modulation [30,31,32,33,34]. The clinical impact of nucleic acid nanocarriers has been clearly demonstrated by the recent COVID-19 vaccines developed by BioNTech and Moderna, which utilize lipid nanoparticles for the delivery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen-encoding messenger RNAs (mRNAs) [35]. Along the same lines, nanocarriers have gained much attention in recent cancer immunotherapy research for the delivery of immunological agents [4]. It has been well documented that nanocarriers can be preferentially delivered into tumors by passive targeting via enhanced permeation and retention effects or active targeting by functionalization with tumor affinity ligands [36, 37]. Similarly, nanocarriers can efficiently accumulate in lymphoid organs and other immune target sites owing to their size effect and can be further engineered to increase their affinity with target immune cells [37]. For example, nanoparticle formulation markedly enhanced the local delivery of immunoadjuvants into peripheral lymphoid organs while restricting systemic distribution, which maximizes their immunological activity and minimizes systemic immunotoxicity [38, 39]. In addition, nanoparticle-based delivery of antibodies effectively targeted and stimulated APCs and T cells and showed clinical benefits in various tumors, such as melanoma, non-small cell lung cancer, renal cancer, and Hodgkin’s lymphoma [40, 41]. Nanocarriers have been designed to directly target and eliminate tumors in the traditional nanomedicine regime, where antitumor efficacy has been critically limited by the systemic and local barriers that hamper tumor infiltration and subsequent cellular uptake of systemically administered nanoparticles [37]. In contrast, they can be directed to immune cells in lymphoid organs or other immune target sites independent of and far from tumors to stimulate systemic antitumor immunity when applied for immunotherapy and, therefore, can bypass the substantial barriers associated with the tumor targeting. Moreover, immune cells exhibit a remarkable ability to permeate through the body’s natural barriers, such as tumor vasculature and the blood–brain barrier, to which nanoparticles generally have limited access, allowing indirect, yet potentially more efficient, therapeutic action of nanocarriers through the modulation of immune cells [42]. Therefore, nanocarrier-based immunotherapy can pursue a new delivery principle of targeting immune cells along with the direct tumor modulation for strong antitumor activity via multifaceted therapeutic action. General approaches for nanocarrier-based modulation of the antitumor immune responses are presented in Fig. 2.
Cancer vaccines
Cancer vaccines aim to potentiate pre-existing or induce new cytotoxic effector functions of T cells by promoting maturation and antigen presentation of APCs that play a critical role in priming T cells (Fig. 3A) [43]. Various immunoadjuvants and TAAs with different physical forms and biological origins are usually co-administered to effectively activate APCs against target cancer cells [44, 45]. Autologous DC-based sipuleucel-T (Provenge®) is the first therapeutic cancer vaccine approved by the FDA for the treatment of hormone-refractory prostate cancer, and its clinical success has spurred the development of various cancer vaccines using several different classes of immunoadjuvants and TAAs [46]. In particular, many tumors are developed by infection with onco-viruses, and virus-induced tumors can be treated with virus vaccines as the infected cancer cells also express and display viral antigens. For example, the human papilloma virus (HPV) vaccine has reported beneficial effects in preventing vaginal, cervical, and throat cancers [47], and the hepatitis B (HBV) vaccine reduced the risk of hepatocellular carcinoma [48], leading to their clinical use for the indicated tumors. A recent preclinical study demonstrated that virus-like particles (VLPs) consisting of viral coat proteins self-assembled at the nanoscale elicited strong innate and adaptive immune responses and generated potent systemic antitumor immunity against a poorly immunogenic mouse tumor model, highlighting the potential of nanoparticle formulations for improving virus-based vaccines [49]. The nanoparticulate virus vaccine has also been developed for treating infectious diseases, which led to the FDA approval of Epaxal® and Inflexal®―virosomal vaccines in which virions are loaded on the surface of liposomes [50,51,52].
Nanocarriers can be applied to cancer vaccines to achieve efficient and selective delivery of immunoadjuvants and TAAs to APCs in peripheral lymphoid organs or other immune sites. Nanocarriers developed for cancer vaccines can be categorized based on their origin as biogenic, semi-biogenic, or synthetic. Biogenic nanocarriers are originated from biological entities with the representative classes including exosomes, outer membrane vesicles (OMV), and VLPs. They can exhibit the inherent antigenic and/or immunostimulatory activities of their origins, which can be exploited for cancer vaccine applications. Exosomes are secreted from virtually all tumor and immune cells, often with intact membrane proteins and cellular components of their original cells. Tumor-derived exosomes display TAAs on MHC molecules and contain cellular danger signals, and APC-derived exosomes present antigens and provide co-stimulatory signals, which can augment T cell response with cancer vaccine function [53]. OMVs are prepared from the outer membranes of gram-negative bacteria, and therefore, present inherent immunostimulatory activity to innate immune cells and APCs to stimulate antitumor T cells in combination with TAAs [54,55,56,57]. Similarly, VLPs are virus-derived non-infectious nanoparticles that resemble the immunological properties of viruses to activate APCs and induce a durable antitumor T cell response with the co-delivery of TAAs [58, 59]. Semi-biogenic nanocarriers are composed partially of biogenic and synthetic components. They encompass the biocompatibility and low toxicity of biogenic nanocarriers, while synthetic modification allows large-scale manufacturing with tunable functionalization. Cell membrane-coated nanocarriers and endogenous protein-based nanocarriers are prominent in this class. In cell membrane-coated nanoparticles, nanocarriers are covered with membranes derived from tumor or immune cells to harness tumor-specific antigens and homotypic tumor targeting properties for a strong anti-tumor response [60, 61]. Endogenous protein-based nanoparticles, such as albumin-based nanovaccines, have the advantages of extended in vivo half-life and efficient uptake by APCs via neonatal Fc receptors to facilitate targeting and activation of APCs for priming T cells [62]. Similarly, endogenous protein-mimicking nanoparticles, such as synthetic high-density lipoprotein nanodiscs, are promising nanocarriers for cancer vaccine delivery [63, 64]. Notably, the “hitchhiking” approach using lipid micellar nanoparticles that readily dissociate amphiphilic immunoadjuvants and antigens and promote their binding to endogenous albumin proteins upon in vivo administration represents a novel approach that combines in situ biogenic nanocarriers with synthetic materials [65]. The inherent lymph node targeting capability of endogenous albumin proteins allowed markedly improved delivery of vaccines to lymph node-resident APCs, leading to robust priming of T cells for strong antitumor therapeutic efficacy (Fig. 4). Synthetic nanocarriers are purely exogenous in origin and have various compositions, such as polymeric, lipid-based, and inorganic nanoparticles [39, 66,67,68,69,70,71,72,73]. They can be physiochemically modulated and functionalized via synthetic and/or post-synthetic procedures [63, 74]. Lipid nanoparticles composed of cationic lipids have been extensively developed for the delivery of mRNA-based TAAs via electrostatic condensation, owing to their ability to improve in vivo pharmacological profiles and cellular uptake by antitumor immune cells [66,67,68,69]. For polymer nanoparticles, while polymer compositions can be largely variable [39, 66,67,68,69,70,71,72,73], the FDA-approved polylactide-co-glycolide (PLGA) polymer is a popular choice, which has demonstrated efficient vaccine delivery to induce robust T cell responses with alleviated safety and immunotoxic concerns [39, 70]. Several different types of inorganic nanoparticles, including gold nanoparticles, carbon nanotubes, and silica nanoparticles, have been reported to suppress tumor growth via vaccine delivery [75,76,77,78]. In a study, a gold nanoparticle-based spherical nucleic acid formulation markedly increased the immunomodulatory activity of nucleic acids to T cells in both humoral and cellular immune responses [75].

Adapted from reference [65] with permission
In situ lymph node targeting and vaccine delivery via albumin “hitchhiking” approach. A The design of amphiphilic vaccines (amph-vaccines) that form micellar nanocarrier structure with an albumin-binding lipid tail. B Ex vivo fluorescence images of axillary and inguinal lymph nodes taken using fluorescently labeled CpG adjuvants and the corresponding fluorescence intensity at 24 h post-injection. C Immunohistochemistry of inguinal lymph nodes at 24 h post-injection. D Average growth curves of TC-1 tumors in C57BL/6 mice.
In particular, mRNA vaccine presents several advantages compared with other types of vaccine, including the inherent immunogenicity, flexible sequence design, rapid production, low cost, and safety. FDA-approved COVID-19 vaccines by Pfizer-BioNTech and Moderna deliver mRNA encoding SARS-CoV-2 spike protein antigen using cationic lipid nanoparticles (LNPs) composed of neutral phospholipid, cholesterol, polyethyleneglycol (PEG)-lipid, and ionizable cationic lipid for condensation and protection of mRNA in the nano-pocket [79]. LNPs promote the cytosolic delivery of mRNA that is then translated to the encoded antigen to stimulate immune cells and confer antibody-mediated immune protection against the antigen [80]. The great success of mRNA vaccines in the COVID-19 pandemic has also attracted refreshed attention to their applications for cancer vaccine. Recent clinical trials have demonstrated the feasibility and therapeutic potential of mRNA cancer vaccine for personalized immunotherapy. RNA mutanome vaccines encoding personal neo-epitopes induced T cell responses to multiple vaccine neo-epitopes, with two of five metastatic melanoma patients experiencing objective response and one patient complete response after immune checkpoint inhibitor combination [81]. A phase I dose escalation study reported the objective response and safety of neo-epitopes-encoding mRNA vaccine to the patients with melanoma, non-small cell lung cancer, and high microsatellite instability cancers [82]. To date, several mRNA cancer vaccines have entered clinical trials and progressed to the advanced phases mostly using LNPs as a delivery system, reflecting the previously demonstrated clinical impact of nanoparticle formulation for mRNA vaccination [83]. In addition, many preclinical studies are also underway to develop non-lipid-based novel nanocarrier platforms for mRNA cancer vaccine. In a study, cationic cell-penetrating peptides (CPPs) containing amphipathic RALA motif were exploited to condensate mRNA into nanocomplexes, which possess pH-dependent membrane disruptive properties at acidic condition with structural transformation [84]. The nanocomplex facilitated cellular uptake and subsequent endosomal disruption for efficient cytosolic delivery and expression of mRNA in DCs, which induced robust CD8 + T cell response for antigen specific killing of target cells. Notably, hollow nanocapsules mainly composed of pathogenic polysaccharide such as mannan and dextran suggest a promising delivery system to provoke strong innate immune response by mimicking microbial pathogens [85]. Particularly, mRNA-loaded mannan nanocapsules have shown to promote the activation and antigen presentation of DCs in vitro and efficient lymph node draining, DC uptake, and priming of CD4+ and CD8+ T cells for strong antitumor immune response and therapeutic efficacy in vivo.
Cancer vaccine is a clinically relevant cancer immunotherapy that can elicit potent antitumor immune response and establish long-term immune memory to eliminate primary tumors and protect against tumor recurrence and metastasis [86]. Although nanocarriers can improve cancer vaccine to effectively prime tumor-specific T cells, tumor heterogeneity and immunosuppression remain inherent obstacles to its clinical translation. The dynamic evolution of tumors during their progression results in the intertumoral and intratumoral heterogeneity with the accumulation of molecularly diverse cancer cells [87]. Accordingly, tumors harbor multiple cancer cell populations expressing distinct TAAs, making it difficult to produce the effective off-the-shelf cancer vaccine. Recently, neo-epitopes formed by genetic alterations of cancer cells have been employed for personalized cancer vaccine and demonstrated promising clinical outcome in a small cohort of patients [88]. However, the personalized approach requires high cost, technical demand, and long lead time for the identification and manufacturing of tumor-specific neo-epitopes, which need to be addressed in the future with the expansion of technology. In addition, cancer vaccine can impose therapeutic selective pressure to the cancer cells, leading to the resistance to treatment by the expansion of the non-targeted sub-clonal cancer cell populations, where the use of multiple TAAs can potentially mitigate the risk of sub-clonal outgrowth [89]. Last but not least, the immunosuppressive tumor microenvironment significantly hampers the performance and therapeutic efficacy of cancer vaccines, which is particularly profound in the advanced stage tumors that have fully established microenvironments [90]. Cancer vaccination is a complex process with the efficiency depending on multiple parameters including the antigens, immunoadjuvants, carriers, and vaccination routes. Therefore, the parameter-function relationship should be investigated thoroughly and optimized in multi-pairwise combinations to induce strong and durable T cell immunity that can potentially overcome the tumor heterogeneity and immunosuppression in the advanced tumors.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs) block inhibitory signals of T cell activation, thereby amplify antitumor activity and unleash the therapeutic potential of T cells (Fig. 3B). Ipilimumab (Yervoy®) is the first FDA-approved ICI that antagonizes cytotoxic T lymphocyte-associated protein 4 (CTLA-4) expressed on T cells for the treatment of metastatic melanoma [91]. The programmed cell death 1/programmed cell death-ligand 1 (PD-1/PD-L1) axis is another important target of ICI, with several marketed products being used in clinics, including nivolumab (Opdivo®), pembrolizumab (Keytruda®), atezolizumab (Tecentriq®), avelumab (Bavencio®), durvalumab (Imfinzi®), and cemiplimab (Libtayo®) for metastatic melanoma, urothelial carcinoma, renal cell carcinoma, and others [92]. There are also other ICIs in pipelines, such as antibodies against lymphocyte activation gene 3 (LAG-3) and T cell immunoglobulin 3 (TIM-3) [93]. However, clinically used ICIs typically show the response only in a fraction of patients (generally 10–30%) who present immunogenic tumors characterized by high number of tumor mutation burden, tumor-infiltrating T cells, and high PD-L1 expression [94,95,96]. Resistance to the treatment and subsequent relapse of tumor have also been sought as limitations of ICIs. Following the therapy, tumor cells can undergo mutations making them less susceptible to MHC response for T cell-mediated killing, TAMs remove therapeutic antibody from the surface of T cells, and T cells upregulate other inhibitory receptors, all of which contribute to the waned response to ICIs and enhanced susceptibility to inhibitory signals [97,98,99]. In addition, since immune checkpoints are crucial for maintaining self-tolerance under normal physiological conditions, ICIs can cause unwanted immune-related adverse events by breaking immune tolerance and promoting autoimmune response. Clinical data indicated that patients often experience treatment-associated side effects when treated with anti-CTLA-4 and anti-PD-1 antibodies [100]. Among the adverse effects, most common are anemia, fatigue, dysphagia, neutropenia, hypertension, and lymphopenia [101]. Additionally, interstitial nephritis, inflammatory pneumonitis colitis, and increased levels of aminotransferase may also be seen with rare incidents of death in cases with severe side effects.
Currently, all FDA-approved ICIs are antibody-based drugs engineered to bind and antagonize immune checkpoint receptors. The clinical utility of these antibody-based ICIs has been limited by the low response rates and immune-related adverse events in normal organs, in part due to their non-specific and systemic in vivo distribution [40, 41]. Current research has mostly focused on combining ICIs with other immunotherapy modalities, such as chemoimmunotherapy and cancer vaccine, for potentiating their therapeutic efficiency with synergistic antitumor immune stimulation [44, 45], leaving the inherent poor pharmacological properties of antibody-based ICIs as a persistent issue. Nonetheless, a few studies have demonstrated molecular and structural engineering of ICIs for improving their in vivo performance. One such approach is tethering tumor-specific affinity ligands to the ICIs for enhanced targeted delivery to and retention in tumors. In particular, it has been demonstrated that conjugation with the collagen-binding domain can render αPD-L1 and αCTLA-4 antibodies to efficiently and durably accumulate in tumors after systemic administration, leading to enhanced antitumor efficacy while ameliorating systemic immunotoxicity [102, 103]. Similarly, nanocarriers can improve ICIs by modulating their pharmacological properties to target immune cells in peripheral lymphoid organs; PLGA nanoparticle-based delivery facilitated the accumulation of αPD-1 antibodies in the spleen and subsequent uptake by splenic DCs after systemic administration, and promoted the maturation and activation of splenic DCs, enabling dose titration of ICIs for effector T cell response [104]. One of the adaptive immune resistance mechanisms operating in the tumor milieu is the induction of PD-1 and PD-L1 proteins by immune and tumor cells via transcriptional regulation [105]. Using a similar approach, immune checkpoint inhibition can be performed epigenetically to downregulate immune checkpoint protein expression. In a recent study, lipid bilayer-coated mesoporous silica nanoparticles were loaded with a small-molecule inhibitor of the signaling hub kinase, glycogen synthase kinase-3 (GSK3), which was designed to interfere with the PD-L1/PD-1 axis by suppressing PD-1 expression [106]. The nanoparticle formulation significantly enhanced drug delivery to the tumor and elicited comparable antitumor efficacy to the benchmark anti-PD-1 antibody, while alleviating treatment-associated toxicity after systemic administration in mouse models of colon, pancreatic, and lung tumors. These studies indicate that ICIs can potentially benefit from nanocarriers that endow locally confined modes of action in tumors and peripheral lymphoid organs via targeted delivery to increase therapeutic efficacy and decrease systemic side effects, regardless of the type of ICIs.
Current research on ICIs is mainly focused on the combination therapies with other cancer treatments to improve therapeutic efficacy of one another in a synergistic manner. Although the combination of non-reductant cancer therapies can improve the clinical responses, it can also exacerbate the immune-related adverse events caused by ICIs. Patients receiving combination therapies of ICIs plus chemotherapy, radiotherapy, VEGF, or VEGFR inhibitors usually exhibit aggravated adverse events with higher incidence of side effects than the respective monotherapies [101, 107,108,109]. The combination of different classes of ICIs also induces a higher number of and more severe immune-related adverse events [110]. Since many nanocarrier-based research also employs ICIs in the context of combination therapies, their potential side effects should be carefully investigated in the preclinical studies along with the antitumor immune response for the development of practical ICI-based immunotherapies.
Adoptive T cell therapy
For T cells to effectively engage and kill cancer cells, they should be abundantly present at the tumor sites and maintain cytotoxic activity towards the target cancer cells. Adoptive T cell therapy involves extraction of T cells from the blood or tumors of patients, ex vivo manipulation, and subsequent reinfusion back into the donor patients, which aims to increase the quantity and quality of T cells for sufficient antitumor efficacy (Fig. 3C) [111]. Tumor-infiltrating lymphocytes (TILs), engineered T cell receptor (TCR) T cells, and chimeric antigen receptor (CAR) T cells represent different types of T cells exploited for adoptive T cell therapy [112]. TILs and engineered TCR T cells rely on the cognate antigen recognition mechanism of T cells in the context of MHC molecules to target cancer cells and mount an antitumor immune response. In contrast, CAR T cells target cancer cells via the extracellular single-chain variable fragment (scFv) antibody domain independent of MHC machinery, allowing tailored binding to a wide range of tumor biomarkers by antibody engineering. CAR-T cells have shown remarkable clinical success with Kymriah®, Yescarta®, Tecartus®, Breyanzi®, Abecma®, and Carvytki® approved by the FDA for the treatment of hematological tumors, such as leukemia and B-cell lymphoma [113]. However, they generally exhibit limited efficacy against solid tumors, which remains a major challenge for adoptive T cell therapy.
Nanocarriers have mainly been exploited to stimulate or preserve T cell activity and maintain long-term viability of T cells upon adoptive transfer. T cell “backpacking” with nanoparticles that carry cytokines associated with T cell proliferation and effector function is a notable strategy, which can endow durable T cell activity by the sustained and localized pseudo-autocrine stimulation with minimum systemic exposure of potentially immuno-toxic cytokines. Multilamellar lipid nanoparticles carrying IL-15 super-agonist and IL-21 cytokines [114] and protein nanogels consisting of IL-15 super-agonist crosslinked via reduction-sensitive chemical conjugation [115] have successfully demonstrated the efficacy and safety of T cell “backpacking” strategy (Fig. 5). Similarly, immunoliposomes that deliver small-molecule inhibitors of TGF-β to adoptively transferred T cells have been shown to maintain T cell proliferation and cytotoxicity by inhibiting immunosuppressive TGF-β signaling in the primary T cells [116]. In addition, ex vivo manipulation of T cells can be improved using nanocarriers that deliver various immunostimulatory signals in a spatiotemporally concerted manner. In a study, polyethylene glycol (PEG) hydrogel was crosslinked with integrin-activating peptides and further decorated with gold nanoparticles functionalized with anti-CD3 antibodies, and the resulting PEG nanostructure stimulated ex vivo activation, proliferation, and differentiation of T cells via integrin-mediated cell adhesion and activation by anti-CD3 antibodies [117]. Small interfering RNA (siRNA) and mRNA can also be effectively delivered through T cell-targeting nanoparticles to downregulate immunosuppressive signaling pathways and potentiate in vivo cytotoxic activity of T cells [118]. An interesting recent development is the application of nanoparticles for in situ generation of CAR T cells, which can avoid the complex procedures and costs involved in clinical T cell manufacturing. Surface functionalization of polymeric nanoparticles with T cell-specific antibody and nuclear localization signals enabled the selective and localized delivery of engineered leukemia-targeting CAR genes into T cell nuclei in situ, leading to efficient programming of circulating T cells into anti-leukemia CAR T cells with robust antitumor efficacy [31]. The versatility of the polymeric nanocarrier for in situ modulation of antitumor T cells has been further demonstrated using mRNAs encoding a genome-editing agent for depletion of TCR and those encoding a transcription factor of favorable memory phenotype formation for enhanced performance of the in situ generated CAR T cells [119].
T cell “backpacking” with nanocarriers. A Multilamellar lipid nanoparticles carrying IL-15 super-agonist and IL-21 cytokines were chemically attached on T cells via maleimide-thiol reaction. B In vivo bioluminescence signal of adoptively transferred Pmel-1 T cells (left) and the survival rate of C57BL/6 mice bearing B16 melanoma after adoptive T cell therapy (right). C Synthesis of protein nanogels by reduction-sensitive crosslinking of IL-15 super-agonist cytokines. D In vitro T cell expansion over 12 days after stimulation with anti-CD3/CD28 antibodies. E Average growth curves of B16F10 tumors in C57BL/6 mice, and F the resulting survival rate of C57BL/6 mice.
The first step of adoptive T cell therapy is the extraction of the sufficient number of host T cells for in vitro engineering and expansion [120]. The frequency of endogenous T cells is largely dependent on the tumor antigen burden, proliferative and functional activity of effector T cells, and their tumor homing capability [121]. In addition, it has been observed that T cells often do not persist and are rapidly cleared, which in turn, demands continuous frequent infusions [122]. Host homeostasis normalizes the pool of lymphocytes, and therefore, lympho-depleting pre-conditioning is required to improve the therapeutic outcome of adoptive T cell therapy by removing endogenous T cells and immunosuppressive Tregs. Other than the quantitative problems, in vitro manipulation can impede the functional capability of the T cells if they are improperly programmed with suboptimal engineering and culture conditions [123]. These T cells may be devoid of its effector functions and undergo depletion and exhaustion with limited proliferation and survival. Finally, the infused T cells can be hindered in their functions due to immunosuppressive tumor microenvironment and tumor-produced soluble factors. Therefore, the strategy for increasing the quantity and quality of host T cells before extraction and the optimal protocol for programing of T cells in vitro should be developed in conjunction with effective in vivo pre-conditioning regimen for robust adoptive T cell therapy [124,125,126,127]. In addition, synergistic combinations of immunotherapies can help maximizing the therapeutic potential of adoptively transferred T cells for potentially overcoming tumor-intrinsic immunosuppressive factors, and thus improving the therapeutic outcomes [128].
Oncolytic virus therapy
Oncolytic viruses (OVs) comprise several different families of viruses with diverse structures and gene organizations, which can selectively infect and replicate in tumors, leading to tumor lysis (Fig. 3D) [129]. Many OVs, including parvovirus, myxoma virus, and Newcastle disease virus, display intrinsic tumor-selective replication and lysis capability in response to tumor-specific cues [130]. Alternatively, viruses can be genetically modified by mutation and deletion of viral genes or insertion of transgenes to render specificity towards tumor cells, such as the cases for adenovirus, herpes simplex virus (HSV), and vesicular stomatitis viruses (VSVs) [131]. OVs can mediate antitumor activity via multiple mechanisms associated with both innate and adaptive immunity specific to tumor cells. In principle, tumor lysis can induce immunogenic cell death to promote the release of TAA and immunostimulatory intracellular molecules, which attract and activate APCs, subsequently leading to priming and tumor infiltration of antitumor T cells. Therefore, OVs can serve as in situ vaccines that can trigger potent systemic antitumor immune responses without exogenous TAAs [132, 133]. In addition, OVs can be manipulated as vaccine delivery platforms by utilizing their inherent inflammatory and immunostimulatory properties, as demonstrated by measles virus, polio virus, and vaccinia virus [130]. Based on the promise in preclinical studies, several OVs have been clinically investigated for the virotherapy of tumors, including melanoma, hepatocellular carcinoma, colorectal cancer, and glioma, which led to the FDA approval of the genetically engineered oncolytic HSV-1 (Imlygic®) for the treatment of advanced stage melanoma after it demonstrated ~ 15% complete tumor regression in clinical trials [134,135,136].
OVs are usually delivered by intratumoral administration because they can circumvent the obstacles associated with intravenous administration, including systemic barriers for delivery into the tumors, anti-viral resistance mechanisms, such as neutralizing antibodies and cytokines in the blood stream, and the risk of off-target replication in non-tumor cells, while facilitating high-dose infection of tumor cells by directly injected viruses [129]. However, despite these obstacles, intravenous administration is the preferred route in the clinic as it can enable delivery to multiple organs and metastatic sites using a simple and convenient injection method. One strategy for safe and effective intravenous administration is to deliver OVs using cell carriers that have tumor tropism and shield viruses from systemic recognition [137]. Similarly, nanocarriers can serve as a shelter that blocks viral neutralization and extends in vivo circulation of OVs to increase their bioavailability and bioactivity after systemic administration [129]. In a recent study, hyaluronic acid-based redox-responsive nanohydrogels were developed and co-formulated with two model OVs, Ad[I/PPT-E1A] (DNA virus) and Rigvir® ECHO-7 (RNA virus), to produce OV-loaded nanohydrogels [138]. Encapsulation in the nanohydrogel preserved the stability of OVs and maintained their oncolytic activity against the respective target cancer cells in vitro.
OVs often require genetic modifications to diminish their virulence and, therefore, avoid toxicity and safety concerns. Alternatively, selected components of OVs that involve tumor lysis and antitumor immune responses can be harnessed as a “subunit” OVs to mimic the therapeutic activity of intact OVs without the potential biohazard issue associated with the whole virus infection. Nanocarriers are promising platforms for systemic delivery of the small size “subunit” OVs. For example, VSV matrix protein (VSVMP), one of the five structural proteins of VSV, is the key element for cytopathogenesis of VSV, and tumor-targeting lipid/polymer hybrid nanoparticles delivering VSVMP genes to target tumors significantly inhibited tumor growth by exerting VSV-like antitumor activity, such as induction of apoptosis, inhibition of angiogenesis, and activation of virus-associated signaling pathways, with no evidence of systemic toxicity [139]. Similarly, virus-derived proteins can be exploited as oncolytic anticancer agents. In a previous study, fusogenic protein of infectious salmon anemia virus (ISAV-F) was delivered to B16 tumor cells using chitosan nanoparticles [140]. The expression of fusogenically active ISAV-F protein decreased cell viability in vitro and delayed tumor growth in vivo without altering the lymphoid population in the tumor and spleen, demonstrating the direct oncolytic property of ISAV-F protein.
Oncolytic virus therapy presents the intrinsic challenges associated with the use of virus. Pre-exposure to a common contagious virus by opportunistic infection elicits host immune response and generates immune memory effect against the virus. When the same virus was utilized for oncolytic virus therapy afterward, the pre-established immunity can facilitate rapid neutralization and clearance of the virus before it can commit antitumor activity in the tumors, which greatly hinders the treatment efficacy by diminishing the viral capability to infect and lyse cancer cells [141]. Moreover, a high dose of viral load can be detrimental due to the uncontrolled inflammation, non-targeted infection, and tissue failure [142, 143]. In addition, viral infection and replication produces antigen proteins and other transcription factors that can be potentially presented to MHC I, which causes elimination of the infected cells and viruses through CTL-mediated immune response [144]. This can limit the replication of virus, resulting in the insufficient viral titers for effective infection and lysis of cancer cells. On the other hand, the direct attack of CTLs on the virus-infected cancer cells can remove cancer cells in the same manner by which cancer vaccine targets and eliminates virus-induced tumors, which can be beneficial to further stimulate cancer immunity cycle for strong antitumor immune response by promoting in situ generation of TAAs [145, 146]. Therefore, nanocarrier-based approaches should also consider the strategies to minimize non-specific host immune response against the virus and achieve the optimal balance of virus replication and elimination by cancer cells and immune cells for safe and effective oncolytic virus therapy.
Perspectives
One of the persistent issues in cancer nanomedicine is the rapid non-specific clearance of nanoparticles by the reticuloendothelial system (RES) that comprises a heterogeneous population of phagocytic immune cells; it significantly decreases the bioavailability of nanoparticles at the tumor sites after systemic administration and causes potential off-target toxicity to RES organs, such as the liver and spleen [4]. Accordingly, the majority of cancer nanomedicine research has focused on the engineering of nanoparticles to minimize their interactions with immune cells to maximize their delivery into tumors [37]. Immuno-oncology offers a unique opportunity for turning the traditional obstacle of nanomedicine into a new opportunity where phagocytosis by innate immune cells and APCs in the RES organs plays a vital role in initiating the cancer immunity cycle and promoting the systemic antitumor immune response. In addition, sophisticated engineering of nanoparticles for their size, shape, composition, and/or surface modification allows specific delivery to target cells in a safe and effective manner. The physicochemical modulation of nanoparticles can be further equipped with a specific function for the induction of robust antitumor T cell immunity. For example, nanoparticles can be engineered to provide immune signals for T cell activation and mimic the function of T cell-priming immune cells [147]. In addition, the unique physical and optical properties of nanoparticle platforms can allow the combination of nanomedicine modalities that directly intervene in tumors, such as photothermal therapy and photodynamic therapy, which have the potential to ablate the immunosuppressive tumor microenvironment and sensitize tumors to immunotherapy [148]. Similarly, the combination of multiple cancer immunotherapy approaches with non-redundant mechanisms of antitumor immunity is a promising next development to achieve synergistic antitumor efficacy, which can be facilitated using versatile nanoparticle platforms capable of integrating multiple immunomodulatory activities in a spatiotemporally controlled manner.
Ideal nanocarriers for clinical cancer immunotherapy application should allow precise delivery of immunomodulatory signals into target immune cells in a safe and effective manner for the orchestration of host immune system in favor of robust systemic antitumor immunity. The preclinical studies introduced in this review have demonstrated various nanocarrier-based engineering approaches to fulfill these requirements, especially in conjunction with cancer immunotherapy modalities that have already been used clinically, suggesting the promise of their clinical applications. Nonetheless, there are substantial challenges for their successful clinical translation: the complex synthesis and post-modification procedures associated with nanoparticle formulation make it difficult to reproducibly manufacture clinical quality products in a sufficient quantity and increase the cost of treatments; the treatment regimen, such as dose, timing, sequence, and route of administration, should be tailored to individual patients to maximize efficacy and minimize toxicity [149, 150]. In particular, exogenously produced and processed nanocarriers always pose a potential safety issue; the biohazard factors associated with nanoparticle formulation have not been defined, and comprehensive safety assessment is hard to achieve for each individual nanoparticles and patients, particularly for chronic toxicity developed over a long period of time with repeated administrations. Although the toxicology of some elements such as heavy metals is well defined in bulk quantities [151,152,153,154], nano-manipulation can change their physicochemical and electrical properties that are potentially associated with biological toxicity [155, 156]. Moreover, toxicology of nanoparticles depends on diverse factors such as size, shape, composition, surface modification, propensity to agglomerate, systemic exposure that are unique to individual nanoparticles [157]. This requires case-by-case investigation of every new nanoparticle entity for their potential toxicity, which is an intensive task that should be performed in a large number of individual patients. Therefore, it is necessary to identify biomarkers associated with the clinical response and carefully select patients who can potentially benefit from the treatment in order to not only avoid unnecessary health risks but also to maximize the therapeutic benefits. Alternatively, it can be a promising approach to repurpose the already-approved nanoparticles with proven safety records as new nanocarriers for immunotherapy, as the decades of enthusiasm have led to the intense development of nanoparticle platforms for cancer nanomedicine, with some of them currently being used clinically. For example, a recent study revealed a hidden intrinsic therapeutic effect of the iron oxide nanoparticle compound ferumoxytol, originally FDA-approved for treating anemia, on subcutaneous adenocarcinomas and liver metastasis [158]. The mechanistic study indicated that ferumoxytol induced the generation of cytotoxic reactive oxygen species coupled to the polarization of TAMs towards a pro-inflammatory M1-like phenotype, suggesting the “off-label” application of ferumoxytol for macrophage-modulating cancer immunotherapy. Since iron oxide nanoparticles exhibit paramagnetic behavior, they can also be exploited for guided delivery of immunotherapy to the target site with the application of an external magnetic field [159]. Most of the previously developed nanoparticles have not been carefully evaluated for their immunological effects because nano-immunotherapy is a relatively nascent field, which provides a sufficient rationale for this approach. In addition, companion diagnostics technique can be useful to develop a new effective delivery system [160, 161]. Although nanocarriers have been studies for their physicochemical parameters and in vitro and in vivo performance, a considerably less attention is paid on how the in vivo behavior of nanocarriers is correlated with the biological system [162, 163]. When nanocarriers are administered into the system, their physicochemical properties can be altered significantly due to the interaction with the biological components, making it difficult to predict the critical factors for in vivo performance [164]. Instead of the point-by-point examination of the nanocarrier parameters, the technique makes use of drug-free version of an intact nanocarrier that is labeled with imaging agents for establishing a relationship between the delivery system and its biological fate in individual patents via advanced imaging techniques, in silico modeling, and bioanalytical methods, which can be used for screening patients who are likely to benefit from the therapeutic version of the nanocarrier [165,166,167]. This approach can allow versatile design of nanocarriers potentially effective to various tumor models, while avoiding the challenges associated with the pinpoint optimization of nanocarrier parameters and identification of patient biomarkers for clinical outcome. Such patient-driven approach can be promising in optimizing and rationalizing nanomedicine for the clinical immuno-oncology [168].
Conclusion
The last decade witnessed a striking clinical success of cancer immunotherapy, which has been emerged as a new pillar of standard cancer treatment. In particular, cancer vaccines, immune checkpoint inhibitors, adoptive T cell therapy, and oncolytic virus therapy have progressed into the clinical applications with individual modes of action to promote tumor immune surveillance and antitumor immune response. Although several different marketed products are currently available for these treatments, they generally have limited antitumor efficacy to benefit only a small subset of patients and cause systemic immunotoxicity. Nanomedicine offers robust and versatile nanocarrier platforms that can potentially address the challenges faced by current cancer immunotherapy and, therefore, improve its clinical outcomes. For example, nanocarriers can allow efficient and selective delivery of cancer vaccines and immune checkpoint inhibitors to lymph nodes and tumors for locally confined immunological activity to maximize their therapeutic efficacy and minimize off-target toxicity. Nanocarriers can also be designed to stimulate and preserve T cell activity and proliferation and enhance the systemic delivery of oncolytic viruses and their subunit components, which have great potential to improve adoptive T cell therapy and oncolytic virus therapy, respectively. The preclinical studies introduced in this review have demonstrated the promise of nanocarrier-based engineering approaches to potentiate the performance of clinical cancer immunotherapy modalities. However, the clinical translation of nanocarriers faces significant challenges associated with manufacturing feasibility, treatment optimization in real patients, and potential toxicity concerns. In this regard, repurposing of nanoparticles previously approved and used for other indications with a track record of clinical manufacturing and safety can be a promising approach to streamline the development of new nanocarrier platforms for cancer immunotherapy. Continuous efforts to develop sophisticated nano-immunotherapy for clinical applications present a significant opportunity not only for nanomedicine but also for immunotherapy to fulfill their full potential for clinical impact, which may lead to a new revolution in immuno-oncology.
Availability of data and materials
Not applicable.
References
Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68.
Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–8.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Oldham RK. Cancer biotherapy: more than immunotherapy. Cancer Biother Radiopharm. 2017;32(4):111–4.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9.
Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–7.
Ventola CL. Cancer immunotherapy, part 3: challenges and future trends. Pharm Ther. 2017;42(8):514–21.
Janeway Jr CA, et al. The complement system and innate immunity, in Immunobiology: The Immune System in Health and Disease. 5th edition. Garland Science; 2001.
Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625(1–3):41–54.
Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol. 2015;42(4):523–38.
Li J, et al. The role of Toll-like receptor 4 in tumor microenvironment. Oncotarget. 2017;8(39):66656–67.
Karapetyan L, Luke JJ, Davar D. Toll-like receptor 9 agonists in cancer. Onco Targets Ther. 2020;13:10039–60.
Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006.
Merino M, et al. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomedicine. 2019;17:13–25.
Qi SS, et al. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017;24(1):1909–26.
Xie Q, Ding J, Chen Y. Role of CD8(+) T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm Sin B. 2021;11(6):1365–78.
Miao L, et al. Transient and local expression of chemokine and immune checkpoint traps to treat pancreatic cancer. ACS Nano. 2017;11(9):8690–706.
Chulpanova DS, et al. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front Cell Dev Biol. 2020;8:402.
Zhang Y, Schmidt-Wolf IGH. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J Cell Physiol. 2020;235(12):9291–303.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Kapadia CH, et al. Nanoparticulate immunotherapy for cancer. J Control Release. 2015;219:167–80.
Jiang W, et al. Designing nanomedicine for immuno-oncology. Nat Biomed Eng. 2017;1:0029.
Barman P, Sharma S, Saini A. Chapter 19 - Improving the functionality of a nanomaterial by biological probes. In Photophysics and nanophysics in therapeutics, N.M. Mahajan, et al., Editors. Elsevier; 2022. p. 379–418.
Akinc A, Battaglia G. Exploiting endocytosis for nanomedicines. Cold Spring Harb Perspect Biol. 2013;5(11):a016980.
ud Din F, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12:7291.
Vaughan HJ, Green JJ, Tzeng SY. Cancer-targeting nanoparticles for combinatorial nucleic acid delivery. Adv Mater (Deerfield Beach, Fla). 2020;32(13):e1901081–e1901081.
Song Q, et al. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017;17(10):6366–75.
Smith TT, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12(8):813–20.
Aalinkeel R, et al. Nanotherapy silencing the interleukin-8 gene produces regression of prostate cancer by inhibition of angiogenesis. Immunology. 2016;148(4):387–406.
Liu L, et al. A novel galactose-PEG-conjugated biodegradable copolymer is an efficient gene delivery vector for immunotherapy of hepatocellular carcinoma. Biomaterials. 2018;184:20–30.
Lai I, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6(1):125.
Fang E, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther. 2022;7(1):94.
Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50.
Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014.
Lynn GM, et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33(11):1201–10.
Kim H, et al. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–8.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61.
Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–43.
Cai J, et al. Improving cancer vaccine efficiency by nanomedicine. Adv Biosyst. 2019;3(3):e1800287.
Barinov A, et al. CD4/CD8/Dendritic cell complexes in the spleen: CD8+ T cells can directly bind CD4+ T cells and modulate their response. PLoS ONE. 2017;12(7):e0180644.
Kawai M, et al. DNA-loaded nano-adjuvant formed with a vitamin E-scaffold intracellular environmentally-responsive lipid-like material for cancer immunotherapy. Nanomedicine. 2018;14(8):2587–97.
Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2018;14(10):907–17.
Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines (Basel). 2020;8(3):391.
Chang MH. Hepatitis B virus and cancer prevention. Recent Results Cancer Res. 2011;188:75–84.
Lizotte PH, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11(3):295–303.
Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008;7(8):1141–50.
Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine. 2002;20(Suppl 5):B17-23.
Ventola CL. Cancer immunotherapy, part 1: current strategies and agents. Pharm Ther. 2017;42(6):375.
Lee YS, et al. Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med. 2011;43(5):281–90.
Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1523.
Lin LC, et al. Advances and opportunities in nanoparticle- and nanomaterial-based vaccines against bacterial infections. Adv Healthc Mater. 2018;7(13): e1701395.
Kim OY, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8(1):626.
Hu Q, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4):2732–9.
Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4(1):5–12.
Baslé E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem Biol. 2010;17(3):213–27.
Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–7.
Fang RH, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–8.
Zhu G, et al. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano. 2017;11(3):2387–92.
Kuai R, et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16(4):489–96.
Kuai R, et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4(4):eaao1736.
Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–22.
Oberli MA, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–35.
Kranz LM, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401.
Bacon A, et al. Induction of a cytotoxic T lymphocyte (CTL) response to plasmid DNA delivered via Lipodine liposomes. J Liposome Res. 2002;12(1–2):173–83.
Geall AJ, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A. 2012;109(36):14604–9.
Rietscher R, et al. Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity. Eur J Pharm Biopharm. 2016;102:20–31.
Qiu F, et al. Poly (propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials. 2018;182:82–91.
de Titta A, et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci U S A. 2013;110(49):19902–7.
Han HD, et al. In vivo stepwise immunomodulation using chitosan nanoparticles as a platform nanotechnology for cancer immunotherapy. Sci Rep. 2016;6:38348.
Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10(3):243–51.
Radovic-Moreno AF, et al. Immunomodulatory spherical nucleic acids. Proc Natl Acad Sci U S A. 2015;112(13):3892–7.
Ahn S, et al. Gold nanoparticles displaying tumor-associated self-antigens as a potential vaccine for cancer immunotherapy. Adv Healthc Mater. 2014;3(8):1194–9.
Hassan HA, et al. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials. 2016;104:310–22.
Xia Q, et al. Functionalized multi-walled carbon nanotubes for targeting delivery of immunostimulatory CpG oligonucleotides against prostate cancer. J Biomed Nanotechnol. 2018;14(9):1613–26.
Schoenmaker L, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586.
Evans MB, et al. COVID-19 vaccine and infertility: baseless claims and unfounded social media panic. Fertil Steril. 2021;23.
Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–6.
Burris HA, et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J Clin Oncol. 2019;37(15_suppl):2523–2523.
Chen J, Chen J, Xu Q. Current developments and challenges of mRNA vaccines. Annu Rev Biomed Eng. 2022;24(1):85–109.
Udhayakumar VK, et al. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv Healthc Mater. 2017;6(13).
Son S, et al. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 2020;20(3):1499–509.
Liu J, et al. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94.
Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–82.
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–60.
van der Burg SH, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–33.
Sun L, Chen L, Li H. Checkpoint-modulating immunotherapies in tumor treatment: targets, drugs, and mechanisms. Int Immunopharmacol. 2019;67:160–75.
Bernard-Tessier A, et al. Atezolizumab (Tecentriq(®)): activity, indication and modality of use in advanced or metastatic urinary bladder carcinoma. Bull Cancer. 2018;105(2):140–5.
Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45.
Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99.
Zaretsky JM, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–29.
Gao J, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–5.
Koyama S, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7(1):1–9.
Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Zhou X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265–74.
Ishihara J, et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci Transl Med. 2019;11(487):eaau3259.
Mansurov A, et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng. 2020;4(5):531–43.
Ordikhani F, et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight. 2018;3(20).
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Allen SD, et al. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials. 2021;269:120635.
Wang Y, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–19.
Wu S, et al. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21(8):1381–9.
An MM, et al. Incidence and risk of significantly raised blood pressure in cancer patients treated with bevacizumab: an updated meta-analysis. Eur J Clin Pharmacol. 2010;66(8):813–21.
Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33.
Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60.
Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250–2250.
Dai H, et al. Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. 2016;108(7):djv439.
Stephan MT, et al. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–41.
Tang L, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36(8):707–16.
Zheng Y, et al. Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors. ACS Nano. 2017;11(3):3089–100.
Guasch J, et al. Integrin-assisted T-cell activation on nanostructured hydrogels. Nano Lett. 2017;17(10):6110–6.
Wayteck L, et al. Comparing photoporation and nucleofection for delivery of small interfering RNA to cytotoxic T cells. J Control Release. 2017;267:154–62.
Moffett HF, et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat Commun. 2017;8(1):389.
Maus MV, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nat Biotechnol. 2002;20(2):143–8.
Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2(8):547–56.
Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–44.
Cheever M, et al. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med. 1982;155(4):968–80.
Kedl RM, et al. CD40 stimulation accelerates deletion of tumor-specific CD8+ T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci. 2001;98(19):10811–6.
Tuma RA, et al. Rescue of CD8 T cell–mediated antimicrobial immunity with a nonspecific inflammatory stimulus. J Clin Investig. 2002;110(10):1493–501.
Kieper WC, et al. Il-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166(9):5515–21.
Yajima T, et al. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168(3):1198–203.
Zhu Eric F, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27(4):489–501.
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy. 2013;5(11):1191–206.
Cattaneo R, et al. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6(7):529–40.
Miller CG, Fraser NW. Role of the immune response during neuro-attenuated herpes simplex virus-mediated tumor destruction in a murine intracranial melanoma model. Can Res. 2000;60(20):5714–22.
Bartlett DL, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12(1):1–16.
Raman SS, Hecht JR, Chan E. Talimogene laherparepvec: review of its mechanism of action and clinical efficacy and safety. Immunotherapy. 2019;11(8):705–23.
Bommareddy PK, et al. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am J Clin Dermatol. 2017;18(1):1–15.
Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–71.
Nakashima H, Kaur B, Chiocca E. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21(2–3):119–26.
Deng S, et al. Development of a new hyaluronic acid based redox-responsive nanohydrogel for the encapsulation of oncolytic viruses for cancer immunotherapy. Nanomaterials. 2021;11(1):144.
Luo L, et al. Targeted nanoparticle-mediated gene therapy mimics oncolytic virus for effective melanoma treatment. Adv Func Mater. 2018;28(29):1800173.
Robles-Planells C, et al. Chitosan-based nanoparticles for intracellular delivery of ISAV fusion protein cDNA into melanoma cells: a path to develop oncolytic anticancer therapies. Mediators Inflamm. 2020;2020:8680692.
Cheng F, Gabrilovich D, Sotomayor EM. Immune tolerance in breast cancer. Breast Dis. 2004;20(1):93–103.
Gahéry-Ségard H, et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72(3):2388–97.
Health NIo and Committee RDA. NIH report: assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13(1):3–13.
Neff-LaFord HD, Vorderstrasse BA, Lawrence BP. Fewer CTL, not enhanced NK cells, are sufficient for viral clearance from the lungs of immunocompromised mice. Cell Immunol. 2003;226(1):54–64.
Davis JJ, Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med. 2005;7(11):1380–9.
Palomba ML, et al. CD8+ T-cell–dependent immunity following xenogeneic DNA immunization against CD20 in a tumor challenge model of B-cell lymphoma. Clin Cancer Res. 2005;11(1):370–9.
Lambert LH, et al. Improving T cell expansion with a soft touch. Nano Lett. 2017;17(2):821–6.
Nam J, et al. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4:398–414.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–51.
Schumacher TN, Kesmir C, van Buuren MM. Biomarkers in cancer immunotherapy. Cancer Cell. 2015;27(1):12–4.
Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup Med. 2006;56(5):307–11.
Vega-Villa KR, et al. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–38.
Kagan VE, Bayir H, Shvedova AA. Nanomedicine and nanotoxicology: two sides of the same coin. Nanomed Nanotechnol Biol Med. 2005;1(4):313–6.
Nel A, et al. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7.
Lanone S, Boczkowski J. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med. 2006;6(6):651–63.
Medina C, et al. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552–8.
Wagner AJ, et al. Cellular interaction of different forms of aluminum nanoparticles in rat alveolar macrophages. J Phys Chem B. 2007;111(25):7353–9.
Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–94.
Chiang CS, et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat Nanotechnol. 2018;13(8):746–54.
Kiessling F, et al. Nanoparticles for imaging: top or flop? Radiology. 2014;273(1):10.
Chow EK-H, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5(216):216–216.
Lammers T, et al. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–87.
Prabhakar U, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncologyEPR effect and nanomedicine drug delivery in oncology. Can Res. 2013;73(8):2412–7.
Lammers T, et al. Personalized nanomedicinepersonalized nanomedicine. Clin Cancer Res. 2012;18(18):4889–94.
Lammers T, et al. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm. 2010;7(6):1899–912.
Miller MA, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med. 2015;7(314):314ra183-314ra183.
Karathanasis E, et al. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology. 2009;250(2):398–406.
Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomed Nanotechnol Biol Med. 2008;4(2):167–71.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C1006643, 2022R1A4A3026347, and 2022R1F1A1075064), Chonnam National University (Grant number : 202233910001), and INHA UNIVERSITY Research Grant.
Author information
Authors and Affiliations
Contributions
Isra Rana and Jutaek Nam contributed to the conceptualization, design, drafting, and writing of the manuscript. Sejin Son contributed to writing and editing of the manuscript. Jaeeun Oh and Juwon Baig aided in the figures. Jeong Hyun Moon contributed to the revision. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, I., Oh, J., Baig, J. et al. Nanocarriers for cancer nano-immunotherapy. Drug Deliv. and Transl. Res. 13, 1936–1954 (2023). https://doi.org/10.1007/s13346-022-01241-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01241-3